Vapor lamps (sodium, mercury and neon) use electricity passing through a low pressure gas to make light. This is done at a much lower temperature than an incandescent light bulb, and the overall color may appear eerie. When you pass this light through a prism, you get a completely different looking spectrum called an emission spectrum or bright line spectra.
![]()
A "bright line" spectrum of hydrogen.
If you were to replace the vapor tube with another element, you would also see a bright line spectrum, but the line pattern would be different. For example, an electrified tube of low pressure helium would produce the following spectra:
A "bright line" spectrum of helium.
Click here to see a YouTube video of spectral lines of different elements
Each element on the periodic table will produce a unique spectral pattern (provided it is under conditions which allow it to produce an emission spectra). This can be a valuable tool for astronomers because this spectral signature can be detected from great distances. For example, astronomers find several places where lots of low pressure gases collect and emit radiation. These places are known as nebulae and can be produced several different ways.
|
A stellar nursery - The Orion nebula Credit & Copyright: MPG/ESO 2.2-m Telescope |
A stellar funeral - gasses expelled from The Little Ghost nebula Credit: Hubble Heritage Team |
When astronomers pass light from these nebulae through their spectrometers (devices which act like prisms), they see emission lines (bright lines). By a process of elimination, they are able to identify all the chemical elements which form the nebula.
How is this all accomplished? First an atom needs to receive some energy from its environment; then it has to release it in the form of light (or another form of electromagnetic radiation).
In a lab, you can "excite" the atoms to emit light by passing high voltage electricity through a tube of low pressure gas, but this is not the only way to accomplish the goal. Atoms in a gas can be energized to emit light by high temperatures. Some of the random kinetic energy of the atoms (atoms just moving and bouncing off other atoms) may be enough to produce light. Atoms can even get their energy from light passing through them. That is, light can be the energy source necessary to compel an atom to emit light.
Before we get too far ahead of ourselves, we have to back off a moment and understand a little about the nature of atoms. You should already know that atoms consist of protons, neutrons, and electrons. You might know that the protons and neutrons reside in the very center of the atoms (known as the nucleus), and that the electrons orbit this nucleus. What you probably don't know is that those electrons orbiting the nucleus have to obey several "rules" imposed on them by nature. Since hydrogen is the simplest atom (only one proton and one electron), it is best if we limit ourselves to this atom (because the story gets more complicated as you add more particles to the atom).
Below is an animation which shows the energy levels the electron is allowed to orbit in (black circles). If the atom absorbs energy from its environment, the electron moves to a higher orbit. When it drops back down, the atom emits a photon of radiation.
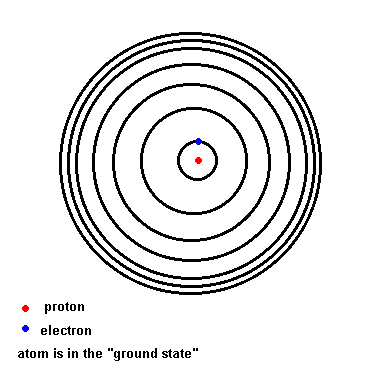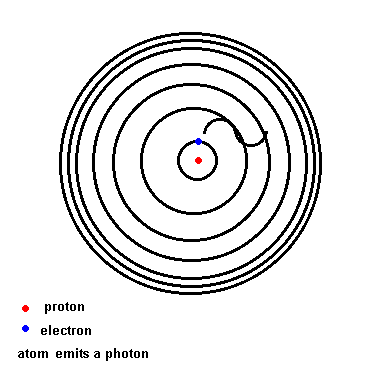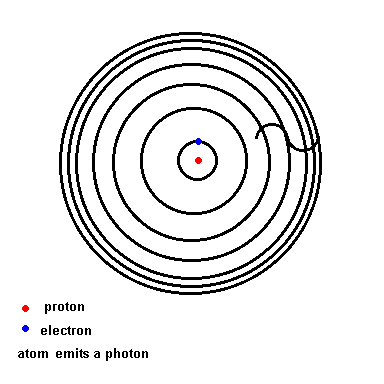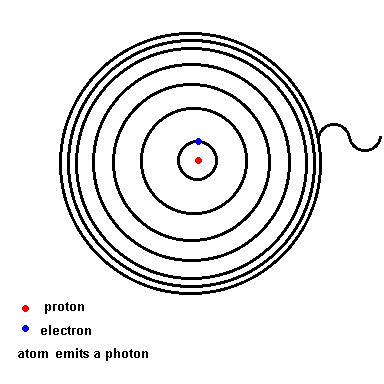
(animation)
This transition, from orbit 2 down to orbit 1, is such a large energy transition that this photon lies in the ultraviolet.
Why do you see only 3 bright lines in the spectra of hydrogen?
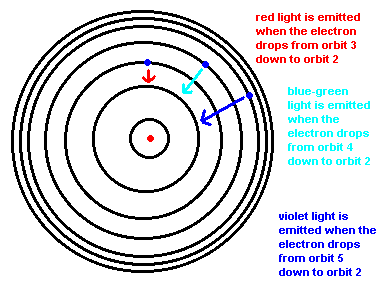
Why we see 3 colors in the spectrum of hydrogen.
![]()
A "bright line" spectrum of hydrogen.
|
Animation showing how hydrogen makes red light. |
Animation showing how hydrogen makes blue-green light. |
To make matters worse, there exists a third type of spectra, known as an absorption or dark line spectra. In order to make this kind of spectra, two things have to happen at the same time.
There needs to be some object capable of producing a continuous spectra.
A low pressure gas has to lie somewhere between you and the object making the continuous spectra.
Dark line spectra of hydrogen
The dark line pattern is exactly the same as the element's bright line pattern. That is, hydrogen gas will produce 3 dark lines which are found where the red, blue-green, and violet bright lines are found in the emission spectra of hydrogen (like a reverse image). Although an absorption spectra is the most complicated, it is also the most important because all stars (including our sun) produce this kind of spectra.
You already know that a background light source is needed. This background source must be producing a continuous spectra (like the blackbody curves studied in the last section). Think of that background light source as an "energy source" for the rest of this discussion. This background light source has a wide variety of colors but also a wide value of energies. Red photons have the lowest energy, and violet photons have the highest energy.

Now let's think about the low pressure gas between you and this "energy source". The electrons orbiting the atoms of this low pressure gas are (if left alone) in the ground state. However, they are not left alone. There are energetic photons passing through. These atoms are now in a situation where they can selectively absorb some of this available energy. And what do they do when they grab this energy? They use it to send electrons to higher orbits. When electrons move down from higher orbits, the atoms emits photons ... however, when the atom absorbs energy, the electrons move up to higher orbits. It should be obvious why the atoms can not absorb just any photon. It can only grab photons with the right energy ... the energy required to move it from one orbit to another orbit .... no more ... no less.
So what do you see? You see all the photons from the "energy source" that the low pressure gas was unable to absorb. You see a dark line spectrum!
Click here to see a great YouTube video that covers all this.
All stars, including our sun, produce absorption spectra.
The solar spectra looks like this
The solar layer known as the photosphere is responsible for producing the background continuous spectrum, but what causes the dark lines? The intermediate low pressure gases could come from two different sources ... our atmosphere or the sun's upper atmospheric layers. As a courtesy, all absorption lines produced by the earth's atmosphere are generally airbrushed out, so you can concentrate on the gases surrounding the sun (or other stars).
In 1802, Wollaston discovered dark lines in the solar spectrum, and in 1817, Fraunhofer rediscovered them. He noticed stars produced different spectral line patterns, but was unable to explain why this occurred.
These spectral absorption lines give astronomers important clues about a star's chemical makeup (and as you will see later, many other pieces of information). For example, the element helium was discovered by noticing that a set of spectral lines of an unknown element appeared in the solar spectrum. It wasn't until later that the same element was found present on Earth. Helium is named for Helios (the Greek sun god).
Click here to view a spectra similar to the sun.
When we covered eclipses, we also discussed the method of discovering planets around other stars as they transit the photosphere. If those planets have an atmosphere, we would be able to detect it because new absorption lines would appear in the spectra during the transit. That is, some of the starlight would have to pass through the planet's atmosphere, ... creating dark lines during the transit. The type of absorption lines would identify which elements make up the atmosphere. Astronomers have already found planets with an atmosphere using this method - click here for details.
Imagine astronomers detect strong lines of oxygen and ozone (O3), you may conclude that the planet has some form of life which uses photosynthesis as an energy source. Ask a biologist for the details. The discovery of life beyond Earth is a very real possibility in your lifetime.
A transit of Venus
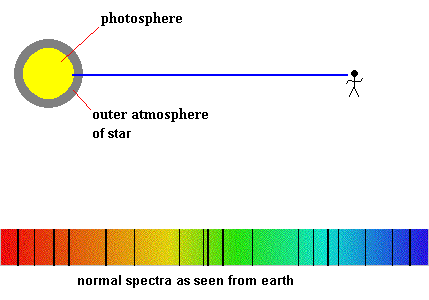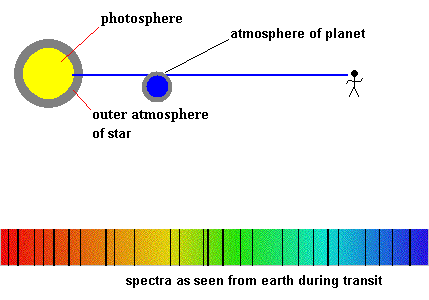
Detecting planetary atmospheres during a transit (animation)
ŠJim Mihal 2004, 2014- all rights reserved