Isaac Newton
courtesy courtesy http://microgravity.msfc.nasa.gov/education/WhatisMicrogravity/WhatMicro.htm
Christiaan Huygens
courtesy imagine.gsfc.nasa.gov/
Images/people/Huygens.gif
Astronomers have a disadvantage in many respects because they are forced to observe the universe from a distance. That is, much of the information they obtain originates from data which is "delivered" to them from the distant corners of the universe. For example, they do not have the freedom to directly sample small pieces of a remote galaxy, an exploding star, or some of the planets (we are making some progress here). Much of the information astronomers get comes from the light and radiation the various objects emit into space. It therefore becomes very important to understand the nature of this radiation so we can dig out the clues it contains.
In the 17th century two great scientists, Newton and Huygens, argued this point (to no definitive conclusion). Newton argued that light was a particle and Huygens contended that it was a wave (much like the waves you find in water). In 1801, Thomas Young showed, conclusively, that light behaved like a wave. A little over a century later, quantum theory showed that light has properties of a particle. As it turns out, modern physics has shown that light does, indeed, have properties of both waves and particles.
|
courtesy courtesy http://microgravity.msfc.nasa.gov/education/WhatisMicrogravity/WhatMicro.htm |
courtesy imagine.gsfc.nasa.gov/
Images/people/Huygens.gif |
When Isaac Newton passed white light through a prism, it separated into a "rainbow" known as the visible spectrum. Since one color blended into another without any gaps, this has also been called a continuous spectrum.
A continuous spectrum from white light
Light is a wave ... called electromagnetic radiation. It consists of an oscillating electric and magnetic field that travels through empty space. This radiation moves incredibly fast in a vacuum ... 186,000 miles each second. The speed of light is designated by the letter c and all the colors in the visible spectrum travel (in a vacuum) this same speed. What, then, distinguishes one color from another? One answer is the wavelength, designated by the Greek letter lambda - λ. This is the distance between successive wave crests. Red light has a slightly longer wavelength than blue light.
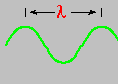
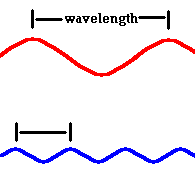
Red light (at one end of the visible spectrum) has a longer wavelength than blue light. However, another way of distinguishing between the different colors of light is by their frequency, that is, the number of waves that pass by a point every second. The animation below is designed to show that red light has a lower frequency than blue light. Imagine you were able to count the waves as they pass by the vertical line. Since both red and blue light travel at the same speed, you would find more blue waves passing the vertical line each second than red waves because the blue waves are closer together.
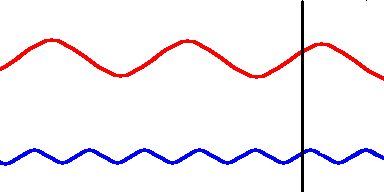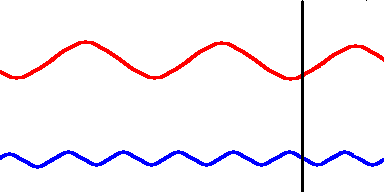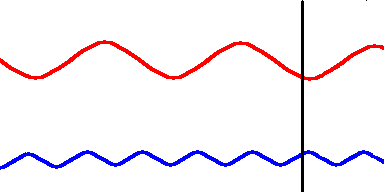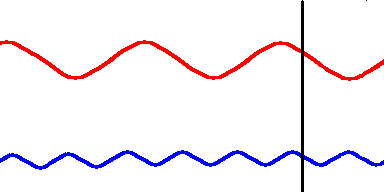
Red light has a lower frequency than blue light. (animation)
The relationship between frequency (f) and wavelength (λ) may be expressed in the following equation:
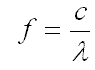
where c is the speed of light. Notice that as wavelength increases, frequency decreases.
But there are waves with higher frequencies (shorter
wavelengths) than blue light and waves with lower frequencies (longer
wavelengths) than red light. These radiations are invisible
to the eye but exist in nature. Together they form the electromagnetic
spectrum.
| Wave type | Wavelength | Frequency |
| radio | very long - several meters | very low |
| microwaves | ||
| infrared | ||
| visible | Red light = .0007 mm or 7000 Å | |
| Blue light = .0004 mm or 4000 Å | ||
| ultraviolet | ||
| x rays | ||
| gamma rays | very short (a few Å) | very high |
Note: Å stands for angstrom units. An angstrom is a very small distance. 1 meter = 10,000,000,000 Å
Just beyond the visible spectrum there exists electromagnetic waves which have slightly longer wavelengths than red light, and are known as infrared radiation. Although we can't see this radiation, we can feel it in the form of heat. On a warm summer day you can feel the warmth of the sun on your skin. This is coming from solar infrared radiation. Your own body radiates quite a bit of infrared radiation, which can be detected by "heat scopes". This is often depicted on TV and in the movies where someone can spy on people in the dark with infrared goggles. One of my favorite movies, Predator, tries to convey the fact that the alien is adapted to view an infrared universe (not a visible one).

Your instructor in infrared (an improvement over the visible one)
With a wavelength just slightly shorter than violet, ultraviolet radiation is also invisible to our eyes. Everyone should be aware that the sun emits UV and that wearing UV blocker will protect you from this harmful radiation. All sunburns are caused by UV damage to your skin cells. In fact, you avoid any types of radiation with short wavelengths ... especially prolonged exposure to X Rays and certainly Gamma rays (how do you think the Hulk was made?)
Here are some main points you need to understand:
![]()
A continuous spectrum (the spectrum of white light)
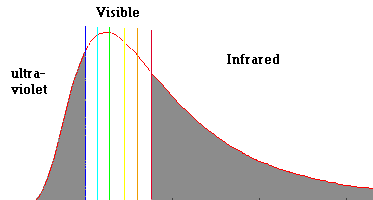
Radiation Curve of the Sun's Photosphere (a hot compressed gas)
Notice that the sun produces roughly the same intensities of all the
spectral colors. This is why the sun looks "white" to the eye ... (do not look
directly at the sun). Also note that the sun produces quite a bit of
radiation in the "invisible" ultraviolet and infrared.
I've found a great source on the web that lets you examine the radiation curves of blackbodies at different temperature. Please spend some time with it so you can discover some important properties of radiators. If nothing appears below, please click on this link.
Please pay attention to two changes as you adjust the temperature - the overall intensity and how the "peak" shifts.
After playing around with the applet above, you will learn some important
properties of radiators. They are:
(T is in Kelvin -see below, λ is in angstroms )
This also gives astronomers a great way of determining exactly how hot a star's photosphere is. By rearranging this equation, you find:
All you need to do is scan the spectrum of a star and determine where it emits the peak radiation (λmax) and apply the formula.
Example: An astronomer scans the spectrum of a star (below) and maps the radiation curve (shown in red). What is the temperature of this star's photosphere? Measurements show that the star puts out the most radiation at 3986 Å. Using Wien's Law, you find:
T = 28,900,000 / 3986 = 7250 K
This also means that the color we see is a function of
temperature. Remember we can only detect the visible with our eyes, so we
are not "seeing" the whole story. Go back to the blackbody
simulator and choose a low
temperature around 3000K.
Now look only at the intensities in the visible. You will discover that
there is a lot more red light produced than blue light. This means we
see a cool star as red.
Now try again with a higher temperature around 5500 K. You will discover that all the colors in the visible are
roughly equal in intensity. This is white light. Note: our sun is
considered a "white hot" star. It appears yellow because our atmosphere
filters out much of the bluer light.
Finally, crank the temperature up to 8700K or "A". You will see that the
star puts out much more blue light than red, ... making it appear blue in
color.
You may have been confused by seeing all the temperatures listed in K. What is a K??? To honor Lord Kelvin, a temperature scale was created where zero point is absolute zero. Absolute zero is the coldest anything can get and it is very cold ... -460 Fahrenheit or -273 Celsius. Don't worry too much about this, but get used to seeing all temperatures listed in Kelvin. Deep space (space far from any stars) is very cold .. only 3 Kelvin. You thought Wisconsin was cold?
Before we finish this section, we need to toss in one more item -
cosmic rays. Until now, all forms of radiation were
electromagnetic in character. That is, a wave of energy that can
travel through space at 186,000 mi/sec in a vacuum. Space is also a
host for high speed particles which can travel near the
speed of light, and under the right conditions, be just as deadly as large
doses of x-rays or gamma rays. Cosmic rays can consist of fast
moving electrons, protons (mostly), or even nuclei of helium atoms (known
as alpha particles). The source of these particles are extremely
energetic events (coronal mass ejections on the sun, exploding stars,
bipolar flow from black holes, etc) ... but fortunately our magnetic field
and atmosphere acts as a shield (or buffer) from this "buckshot" from
space.
©Jim Mihal 2004, 2014- all rights reserved