The Carbon Cycle
(and temperature change)
During most of earth's history, the climate was much warmer than it is today.
Yet the younger sun put out 30% less light than it does today???
How is that possible? The answer is carbon! You have all
heard about the greenhouse effect and global warming. These are issues
that deal with the way humans have been shifting carbon around in our
environment (by burning fossil fuels). It turns out, nature has been doing
the same thing all by itself from the start.
Carbon can take many forms, and the carbon we are referring to is carbon dioxide
(CO2) in our atmosphere. Presently our atmosphere contains very
little CO2 ... only .03%. The atmospheres of both Venus and
Mars are almost completely composed of carbon dioxide.
Carbon (in different forms) is constantly moving between the atmosphere,
oceans, and land in a cycle know as the carbon cycle. It is the focus of
much attention because CO2 (carbon dioxide) is an important
"greenhouse" gas which effect global temperatures. In unit 4 we will
discuss the greenhouse effect in detail. For now, all you need to know is
this - the more CO2 in the atmosphere ... the warmer we get. By burning
fossil fuels, we are releasing CO2 into the atmosphere and making things warmer.
How does carbon enter the atmosphere?
-
Volcanic outgassing
-
Respiration (animals exhale CO2 )
-
Decay of biomass
-
Burning of fossil fuels
-
Weathering of carbonate rocks (limestone)
How does carbon leave the atmosphere?
-
Plants convert atmospheric CO2 to biomass (producing
carbohydrates and oxygen) via photosynthesis. If the plant decays, it
simply puts the CO2 back in the air (using decomposer
bacteria). If it gets buried (in a bog for example), it can not decay and
the CO2 is tied up as biomass which may
eventually get converted to a fossil fuel (coal, oil, or natural gas)
- When we eat the plants, we take in carbon as biomass. This means you
are a part of the carbon cycle. Every breath you take you exhale carbon
and every time you eat something, you are taking carbon in.
-
The chart (below) shows that forests act as a major CO2
reserve. The clearing of the tropical rain forests not only releases
this storehouse of carbon dioxide when it is burned, but the loss of the
trees itself means we've lost another tool for removing additional CO2
for tomorrow.
-
Rain absorbs CO2 and converts it to carbonic acid (H2CO3).
Over geological time, this acid corrodes rocks made of granite. In the first
unit we talked about the weathering of granite - and if you recall, the
continents are mostly granite. This reaction releases the highly resistant SiO2
as sand, also produces calcium and magnesium as ions in solution. The CO2 ends up as bicarbonate in solution (CO3
). The calcium, magnesium, and CO3 then collect in the oceans and build
up. In the oceans they reach saturation levels (near the surface) and begin to
precipitate as calcite, which is calcium carbonate. Ocean-dwelling life-forms
convert the saturated calcium carbonate into body parts such as shells, pearls,
bony structures, and coral. When they die, the animals' parts end up on the ocean floor as carbonate (limestone).
All this "buried" CO2 is then stored as thick layers of
ocean floor sediments. If this layer of the crust is later subducted under
by another plate, the CO2 may be released
back into the atmosphere as volcanic gas.
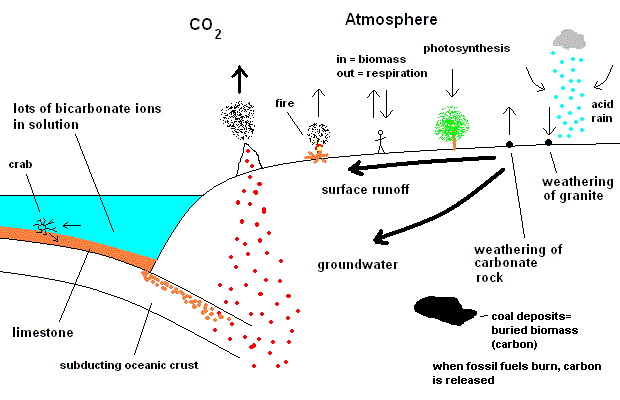
The
Carbon Cycle
Note that carbon dioxide migrates in many cycles ... each working on
vastly different time scales.
Where is all the carbon now (and how much is there)?
| The World's Carbon Reservoirs |
| Reservoir |
Size (Gt C) |
| Atmosphere |
750 |
| Forests |
610 |
| Soils |
1580 |
| Surface ocean |
1020 |
| Deep ocean |
38,100 |
|
| Fossil fuels |
| Coal |
4,000 |
| Oil |
500 |
| Natural gas |
500 |
| Total fossil fuel |
5,000 |
data with permission from http://www.gcrio.org/CONSEQUENCES/vol4no1/carbcycle.html
GT = gigatons A gigaton is a billion (109) metric tons, 1012 kg,
or about 2200 billion pounds.
You can see that most of the carbon reserve is tied up in the deep ocean
but as bicarbonate in solution. Marine organisms can not make use
of it because they thrive near the surface and because minerals such as
calcite (CaCO3) are unsaturated at depths below 4 to 5 km so
it becomes difficult for organisms to use it for making hard parts.
This is because the solubility of CO2 increases as the
temperature drops and pressure increases (and why your soda fizzes when
you release the pressure or warm it up). At unsaturated levels, these
hard parts would dissolve ... leaving the organism defenseless.
The carbon cycle is a thermostat which helps the earth regulate the climate.
-
Too much CO2 in the atmosphere and the earth gets
warm!
-
Too little CO2 in the atmosphere and the earth
cools off!
-
The rate of CO2 reaction to form carbonate (via chemical
weathering of rocks) is strongly dependent on the temperature. If the
temperature goes up, the rate of chemical reaction goes up too, sucking CO2
from the atmosphere very fast. The reduced CO2
levels in the atmosphere then helps cool the atmosphere.
-
If the temperature drops, the rate of chemical reaction slows.
If less CO2 is removed from the atmosphere, the temperature rises.
-
This positive feedback will ensure that the planet's temperature
will stay in a range where liquid water can exist, keeping Earth habitable.
-
The carbon cycle helps explain how the earth could be much warmer in the past
despite the fact that the sun produced 30% less light. There was a lot
more carbon dioxide in the atmosphere when compared to the levels we see today.
Taken from Christopher P McKay, NASA Ames Research Center,
Astronomy
Magazine, September 99 page 92
-
Venus is an example of a planet with a run-away greenhouse effect. Since
it has no water, it has no mechanism to remove CO2 from its
atmosphere. Venus is hot (482 degrees Celsius) because it's closer
to the sun and has a thick atmosphere which is 96% carbon dioxide!
-
The sun (as it ages) slowly gets hotter and hotter. In time, this will
continue to remove more CO2 from the atmosphere.
Eventually the CO2 levels may reach levels so low that plants
may not be able to exist. No plants, no us. Another possibility is
that once the CO2 levels drop to zero, the feedback mechanism
fails and we burn up. Either way, bad news!
On February 20, 2000 a team of astronomers from Penn State painted a
pretty dismal picture concerning the fate of the earth in the next billion
years. Read their press release here.
-
In the short run, we are experiencing global warming.
Is this natural or a result of the burning of fossil fuels (and clear-cutting
the tropical forests)? The data confirms that both are rising.
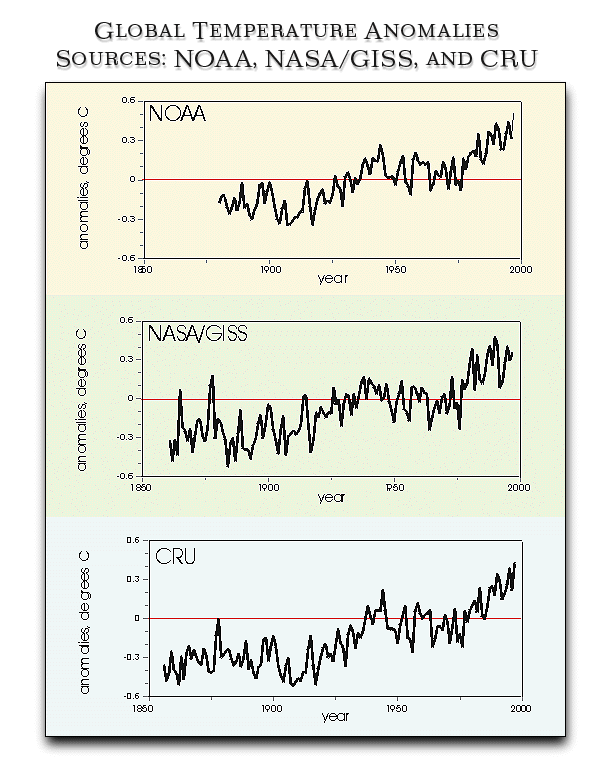
from NOAA
Click here to
see another chart of global temperature change.
Carbon Dioxide levels in the Atmosphere
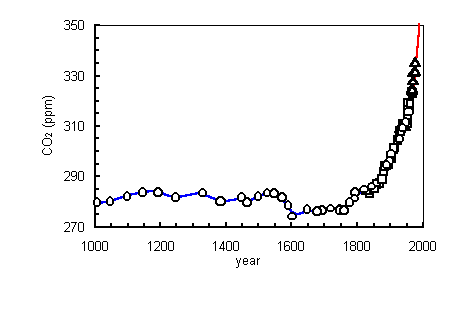
Permission from http://www.bsi.vt.edu/biol_4684/Cycles/Cprobs.html
The major greenhouse gases from human activity and the approximate proportion
of the
human-induced effect are:
| CO2 |
Carbon dioxide |
55% |
| CH4 |
Methane |
20% |
| CFCs |
Chlorofluorocarbons |
18% |
| N2O |
Nitrous oxide |
5% |
Globally, human activities are adding about 26 gigatons (26,000 million
tons) of carbon dioxide into the atmosphere each year, this is only about
3% of the natural flux between atmosphere and oceans or land.
Carbon Dioxide and You
Do you think you only contribute only a very small amount to this increase in
atmospheric carbon dioxide? Guess again! Read
this article and then you will see that you are putting tons of CO2
into the air each year. For example, you emit 25 pounds of CO2 into
the air for every gallon of gas you use ... and a ton of CO2 just
to run your refrigerator for a year! It shows that (on average) each
person is responsible for adding over 8 tons of CO2 into
our atmosphere each year! This article was a real reality check for me
when I read it.
Links to the Carbon Cycle:
http://www.gcrio.org/CONSEQUENCES/vol4no1/carbcycle.html
ŠJim Mihal 2004, 2006 - all rights reserved

