
Strata are layered horizontally at first ...

... then tilted at a later time! I'm waving HI
Portions of bullet list copied (seeking permission) from University of Dublin, Trinity College
Portions of bullet list copied (with permission) from Dr. Pamela
J. W. Gore,
Georgia
Perimeter College at
http://www.gpc.edu/~pgore/geology/geo102/age.htm

Strata are layered horizontally at first ... |

... then tilted at a later time! I'm waving HI |

The Grand Canyon ... can you tell which layers are older?

Igneous intrusions at Gunnison CO.
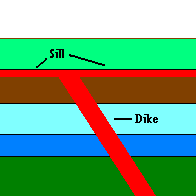
On occasion, a igneous intrusion will cut between sedimentary layers forming a layer of sill. But this is easy to detect because when it forces its way between layers, it will pick up pieces of each layer (above and below) which become embedded in the igneous rock. A dike is an igneous intrusion that cuts across strata and has to be younger than the rock it moves through,
Yosemite National Park is an example of a batholith (a huge magma chamber now exposed to the surface) .... kind of like a giant dike that once displaced existing rock
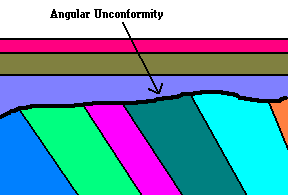
 Can you find the unconformity? Can you find the unconformity?
Both images taken in Utah |
 |
Can you place each layer in the proper time sequence? |

Permission from Miami University |
Here we see an angular unconformity. This tells us an interesting
story.
Now it's your turn to use the principles above to do your own bit of geologic detective work.
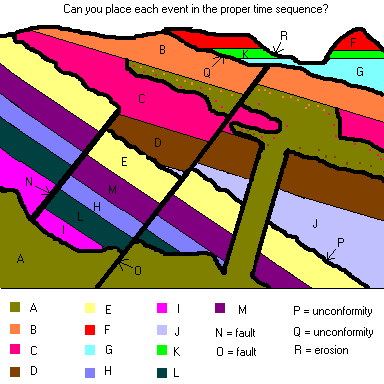
You should be able to tell the "story" how this area evolved to its
present situation. Now, give it the old college try and list the
events ... starting with the oldest. I've listed the correct answer
at the bottom of the page ... but don't peek.
This reminds me of a story. I took my family once to Williamsburg (an old colony which has since been renovated to its original state). There a employee acting the part of a blacksmith held up a coin with a kings image on it and asked the crowd, "Can anyone guess when this coin was minted? (of course, the blacksmith's point was to know who was king of England at that time)" . My son, Adam, who was only about 6 at the time shouted out ... Look at the date stamped on the coin!"
How to get the exact year of an event:
Tree rings (to
determine when fires occurred in the past, times
of drought, etc.)
Glacial Ice (similar to tree rings - get air samples
trapped in the ice, look for pollutants, spores, etc.)
Sediments in some shallow arctic and alpine lakes
(sediments change with the seasons)
How to get good estimates:
Water diffuses into obsidian at a known rate (to date tools used by man).
Growth rates of lichen colonies (the fuzzy green plants that sometimes grow on exposed rock) indicates that the size of the colony increases with time at a predicable rate. Some individual colonies have existed for over 8000 years in arctic regions.
Naturally occurring radioactive materials break down into other materials at known rates. This is known as radioactive decay. Radioactive parent elements decay to stable daughter elements. Once this rate is known, geologists can estimate the length of time over which decay has been occurring by measuring the amount of radioactive parent element and the amount of stable daughter elements.
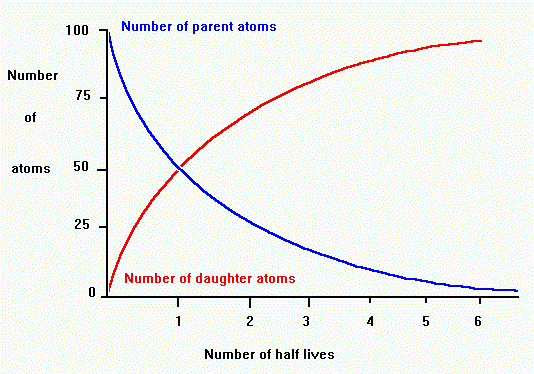
Each radioactive isotope has its own unique half-life.
A half-life is the time it takes for half of the parent radioactive element
to decay to a daughter product. The proportion of parent to daughter tells
us the number of half-lives, which we can use to find the age in years.
For example, if there are equal amounts of parent and daughter, then one
half-life has passed.
Radioactive Parent |
Stable Daughter |
Half life |
|---|---|---|
Potassium 40 |
Argon 40 |
1.25 billion yrs |
Rubidium 87 |
Strontium 87 |
48.8 billion yrs |
Thorium 232 |
Lead 208 |
14 billion years |
Uranium 235 |
Lead 207 |
704 million years |
Uranium 238 |
Lead 206 |
4.47 billion years |
Carbon 14 |
Nitrogen 14 |
5730 years |
There are now over forty different radiometric dating techniques, each based on a different radioactive isotope. Each one is appropriate for a given time interval depending on the half life.
The Carbon 14 test is commonly used to date organic material such as wood and bone. C14 is produced naturally by cosmic rays entering our atmosphere and interacting with atmospheric nitrogen 14. All living organisms take in carbon (mostly carbon 12) but some of that carbon is a small amount of this radioactive isotope (C14). When the organism dies, it can no longer take in additional Carbon 14, so the amount decays over time, producing nitrogen 14 as a daughter product. After 5730 years, half of the C14 is gone, after two half lives (11,460 yrs), 75% of the C14 is gone, etc.
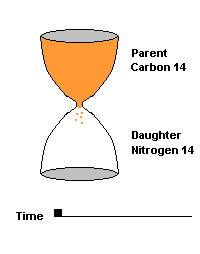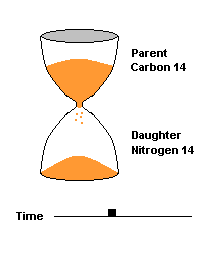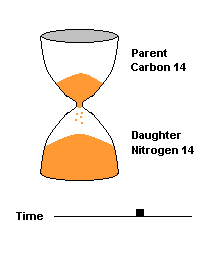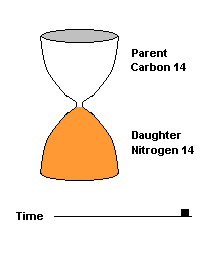 (animation)
(animation)
This hourglass animation is a good analogy to help you understand the principles involved. Suppose you walk into a room that has an hourglass already going. You can estimate how long ago the hourglass was turned by observing the amounts of sand left in the top (parent isotope) and how much has already moved to the bottom (daughter isotope). Of course, you will have to know the basic properties of that particular hourglass (which can be done at a different time) ... does it really take an hour to empty? But once done, you have a pretty good idea how to use this device to estimate the passage of time. The hourglass analogy breaks down at the very end because in theory, there will always be some sand in the top half (parent isotope) of the hourglass.
The carbon 14 test does have limitations. For example, the Carbon 14 test would only be accurate for more recent times ... within 70,000 years (since it has a rather short half life). That is, after about 70,000 years there would be so little C14 left in the sample that it would be difficult to accurately measure. So you can see that you could not, for example, use the Carbon 14 isotope to date something like a dinosaur bone (over 65 million years old) but it would be very appropriate for dating the a piece of wood found in the pyramids. Another problem arises because scientists have to estimate the initial amount of carbon 14 trapped in the body when it dies. The sun does vary in the amount of cosmic rays it produces over time, so the amount of C14 in the atmosphere also varies slightly. These variations are well known over the past 6000 years (because C14 is actually trapped in tree rings) but before that, it has to be estimated.
This technique is only valid for igneous rocks and the event being dated is when the rock was formed (crystallized) from magma or lava. If a rock re-melts, the radiometric clock is reset. Fortunately, there are enough radioactive isotopes with a wide range of half lives so that just about any igneous rock can be accurately dated. And there are several different tests that can be performed on one sample, so that if all the tests provide the same date of origin, you can be reasonably sure that the age is correct.
An Update
On January 11, 2001 scientists claimed that they found evidence of a rock dating 4.4 billion years old. This is, by far, the oldest dated rock (the oldest before this find was about 3.8 billion years). This crystal could only have formed at much lower temperatures than thought possible at this time in earth's history. What was interesting about this find was that there was evidence that liquid water was present at the time of formation. This was quite a surprise and offers clues that the earth had oceans and continents .... and even could have supported life at this very early time! Click here to read the story.
In addition, any given species may inhabit the earth for only a certain lifespan (see image below). For example, geologists may know that species "A" existed for a given range of dates (shown in red) and species "B" for a different range of dates (shown in cyan). Finding sedimentary layers with either species (alone) may only give a rough estimate for a time of formation. However, if a sedimentary layers contains both species, a geologist can narrow the time of formation to a much smaller range of dates (shown in blue). And you thought that Monk was a great detective?
The problem is, fossils are found in sedimentary rocks and generally will only indicate the relative ages of different strata. But sedimentary layers may be "sandwiched" between layers of igneous rocks (which can be dated) and this gives an absolute time when the species lived.
With this in mind, the geologic time scale is set up according to the
life forms which dominated the earth during different eras.
Cenozoic Era - Modern Life, "Age of Mammals" - 0-65 million years ago
Mesozoic Era - Middle Life, "Age of Dinosaurs" - 65 - 250 million years ago
Paleozoic Era - Early Life, "Age of Fishes" - 250 - 570 million years ago
Precambrian Era - Ancient Life - simple organisms with soft bodies (so the fossil record is sketchy)
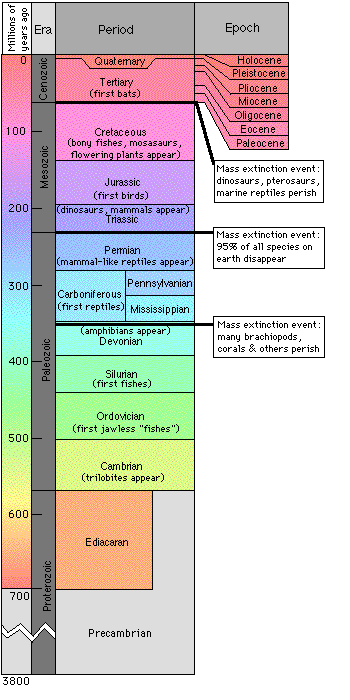
Permission from 2000 Friends of the Geology Museum
As you can see from the diagram, an era covers the greatest expanse of time. Each era is subdivided into blocks called periods. The movie "Jurassic Park" reminds us that dinosaurs ruled the earth during this time. If that isn't enough, each period is subdivided into blocks called epochs. Should anyone ask, we are currently in the Cenozoic era, the Quaternary period, and the Holocene epoch. For reference, the Cenozoic represents the age of mammals (over the last 65 million years), the Quaternary represents the age of man (from the last 1.7 million years), and the Holocene represents the age of civilization (which is approximately the last 10,000 years).
Links
http://www.nmnh.si.edu/paleo/dino/timescal.htm
About geologic time from the USGS
About fossils from the USGS
Answer to puzzle above:
I, L, H, M, E, ... now the area was tilted slightly and eroded producing unconformity P
Now the next part is interesting ... either fault N or layer J came next (you can't really tell from the picture) but a good guess would be fault N because that would be produced when the rock is under great stress. Layer J is created when the area is again undergoing a quiet period of deposition.
Next came D (can you see why N came before D?) ... C, B ..
Now igneous intrusion A and another fault O followed by Q (more tilting and erosion)
Finally G, K, F and ending with R. How did you do? Don't worry,
the test will will never have anything this hard on it.
ŠJim Mihal 2004, 2006 - all rights reserved