The Atmosphere
The earth's atmosphere (without considering water vapor) is mainly
composed of two gasses:
Nitrogen (N2) 78%
-
This really doesn't do much in the atmosphere. It is needed in the ground
by some plants (where it is taken in to the ground as nitrates any time
lightning strikes the ground). This constitutes another "cycle" know
as the
nitrogen
cycle. You learn more about this in a biology class.
Oxygen (O2) 21%
-
You need this to breathe!
-
It is/was produced as a byproduct of photosynthesis.
Earth's very early atmosphere did not have any (measurable) oxygen.
The first life forms on earth did not use photosynthesis (anaerobic bacteria)...
in fact, oxygen was poisonous to them. Photosynthetic bacteria changed all
that by finding a way to use sunlight as an energy source. The byproduct was
oxygen.
-
Without oxygen, there is no ozone, O3 , in the atmosphere.
Without ozone, life would be limited to the sea because ozone filters out much of the
harmful UV radiation from the sun.
The rest is:
Argon (Ar) .9% - This is an inert gas which doesn't really do much.
Carbon Dioxide (CO2) .03%
You already know the importance of this gas and the role it plays in
the carbon cycle. Specifically, it is important because the amount
you find in the atmosphere directly effects:
-
Chemical weathering of rocks
-
Global warming - the greenhouse effect
-
In the last century, global average temperatures have risen 1 degree Fahrenheit.
-
The global average temperature now is 59 degrees F ... with no greenhouse
gasses in the atmosphere (including other gasses such as methane and water
vapor) ... the global average temperature would only be 0 degrees F.
-
The atmosphere of Venus is almost all CO2 and the surface temperature there
is hotter than your oven (in the self clean mode).
-
The debate continues ... how much of the global warming comes from CO2
emissions from the burning of fossil fuels?
Ozone (O3) (.0006%)
-
Mostly concentrated in the part of the atmosphere known as the stratosphere.
This gas filters out much of the suns harmful UV (ultra violet) radiation.
The little amount of UV that does get through can cause sunburn when
you stay on the beach too long. Always use UV sunglasses and use
plenty of UV blocker.
-
Ozone is actually poisonous to us and is "manufactured" near the surface of the earth during lightning strikes
(ever smell a storm?) and as part of a photochemical reaction with fossil
fuel emissions (ozone alerts). It can make breathing difficult and
cause health problems.
Solar Radiation and the Atmosphere
The sun produces more than just light (visible spectrum). It produces
"invisible" radiation such as UV (ultraviolet) and IR (infrared) radiation.
As stated before, UV causes sunburns and should be avoided. Infrared
is sometimes known as "heat radiation" because it is detected by your skin
as a warming sensation. Ever feel warm and cozy under a "heat lamp"?
Scientists know that these types of radiation differ in wavelength (and
designated with the Greek letter lambda ).
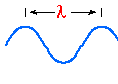
Ultraviolet = short wavelength ... less than
.0004 mm
Visible = wavelength between
.0004 mm (violet) and .0007 mm (red)
Infrared = long wavelengths ... longer than .0007
mm

Red = .0007 mm
Violet = .0004 mm
How much of each type does the sun produce? This is known as a
radiation curve. Click here,
slide the temperature bar to around 6000 and click "new". This will draw a
radiation curve for the sun. It should look something like this:
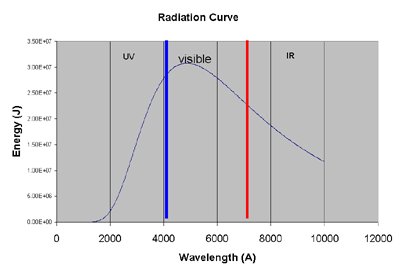
The sun puts out about:
-
44% of its radiation in the visible.
-
7% of its radiation in the UV.
-
37% of its radiation in the near IR.
-
11% of its radiation in the far IR.
-
1% other types (x rays, microwaves, radio).
However, only about 50% of the radiation reaching the
upper atmosphere actually reaches the surface and gets absorbed.
The bulk of this radiation reaching the ground lies in the visible.
About:
-
5% is scattered (back to space) by the air.
-
27% is reflected (back to space) by clouds.
-
20% is absorbed by clouds and the air.
-
3% is reflected off the surface.
The remaining radiation is absorbed by the earth. When radiation
is absorbed, the object absorbing the radiation will heat up, but:
-
Some objects absorb radiation better than other objects. For example,
black objects absorb better than white objects. Also, a red object
appears red because it is absorbing all the colors of the spectrum except
red (which it reflects).
-
Some objects absorb radiation and use it for food (which is why a patch
of green grass doesn't get so hot).
-
Different objects heat up to different temperatures when they absorb radiation.
For example, water will heat up very little when it absorbs radiation
but land heats up quite a bit more (about 5 times more) when it absorbs
the same amount of radiation. A physicists would say that water has a higher specific
heat than land. Also, water will distribute much of the heat
it absorbs to deeper layers because water is transparent (so it doesn't
just absorb at the surface) and can transport heat easily from the surface
to deeper layers by mixing. You may be very familiar with the phrase
"cooler near the lake" on a hot sunny summer day. Now you know why. Because of these properties, large bodies of water can moderate the
climate (produce less extremes) than over land. Land tends to
get very hot in summer and very cold in winter (when compared to water).
This idea is important in understanding many ways water and land can effect
the climate.
So what does an object do when it absorbs radiation? It not
only heats up, but it then re-radiates energy back to the sky ... but at
a much longer wavelength than the type it absorbed in the first place.
In the case of the earth, the radiation coming off the surface is in
the far infrared. But gasses in the atmosphere like CO2, water vapor,
and methane easily absorb this energy and heat up the atmosphere instead.
This is the greenhouse effect! This means that the lower atmosphere
is actually heated from the ground up by radiation emitted by the earth
(and
by conduction/convection originating from the surface).

Because the lower atmosphere is heated from the ground up, the higher
up you go in the atmosphere, the cooler it gets (to a point). On
the average, for every 1000 feet you move up in the atmosphere, the temperature
drops 3½ degrees Fahrenheit. It is easy to see why glaciers
can exist on the tops of mountains.
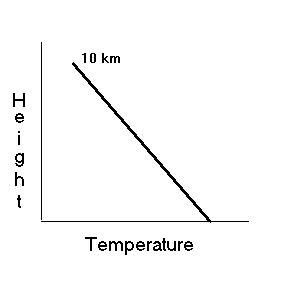
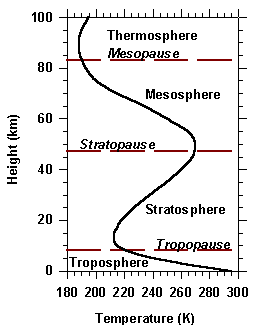
The Troposphere
The lowest layer of the atmosphere is the most important! The troposphere
extends to a height of 10 km (6 miles). Almost ¾ of the entire
atmosphere is found in this layer alone ... including all the interesting
weather we see from day to day. For that reason, the troposphere
is often called the "weather sphere". Typically the temperature
drops with height in the troposphere but not always ... On occasion, it
may actually rise as you move upward ... this is called an inversion.
As stated before, the temperature typically drops 3½ degrees
Fahrenheit for every 1000 feet you move up.
The jet stream (covered later) can be found at the top of the troposphere.
It also marks the boundary for the next layer ...
The Stratosphere
Often called the "Ozone Layer", the stratosphere marks the point were temperatures
start to increase with height. This is because Ultra Violet
(UV) radiation entering the atmosphere from above gets absorbed by the
small amounts of ozone concentrated between 10 - 50 km above the surface.
This is good for us because UV radiation is harmful. But why is
ozone concentrated here?
To make ozone (O3) you need diatomic oxygen (O2)
and monatomic oxygen (O) and lots and lots of tricky collisions.
Monatomic oxygen is found at the very top of the atmosphere but at that
level, the air is so thin that there are not very many collisions ....
(an important ingredient) to make ozone. At the surface the air is
more dense, so many collisions are possible ... but there is not enough
monatomic oxygen to make ozone. Only in the stratosphere is there
the right combination of ingredients and necessary collisions to successfully
manufacture ozone.
Perhaps you heard about the problems
dealing with the "hole"
in the ozone layer mostly over the south pole (in spring) ... but is seems
to be thinning in vast regions as well. It seems to be getting worse!
Is this caused by air pollution from "chloro- fluorocarbons". The
use of these chemicals in spray cans has been outlawed and the type of
chemicals being used in refrigerators and air conditioners is being replaced
with a new kind that is less harmful to ozone.
The top of the stratosphere marks the beginning of:
The Mesosphere
Temperatures again drop with height in this layer which extends from 50 to 80 km
above the surface. Most meteors burn up at this layer.
The last layer is:
The Thermosphere
This layer (like the stratosphere) has a heat source by absorbing UV radiation
from the sun. For that reason, the temperature increases with height.
Permission from CRISP at http://www.crisp.nus.edu.sg/
ŠJim Mihal 2004 - all rights reserved


![]()



