Lead Storage Battery (animation)
Key Terms
battery
electroplating
fuel cell
thermocouple
thermoelectric cooler
As stated in an earlier section, electrically charged particles exert forces between themselves. Particles with the same electric charge repel each other and particles with unlike (opposite) electric charges attract each other. One way an atom acquires an electric charge is when it gains one or more electrons. The atom is then called a negative ion. How is that possible? The answer has to do with the way electrons arrange themselves in the atom. Electrons can't just orbit anywhere they want. They have quantum rules that dictate their energy and position. Electrons surround the atom in "shells" which act like steps. Once a shell is filled, the atom becomes more stable at that point and any additional electrons start filling the next shell. One group of atoms is called the noble gases (a total of six including helium and argon). Chemically these gasses are extremely stable because their outer shells are completely filled (or a full s and p sublevel). For that reason, they do not form chemical bonds easily. All other atoms have shells that are not complete and they attempt to find ways to establish that same stability. One way is to simply gain extra electrons, forming an ion. For example, the chlorine atom is one electron short of filling its outer shell (called the valence shell). By accepting an extra electron, it achieves this stability and becomes a negatively charged ion in the process. Sodium, on the other hand, has one electron in its valence shell (outer shell) and would love to lose it. By shedding this extra electron, the sodium atom becomes a positive ion. Now all you need to do is form a marriage. The positive sodium ion will attract the negative chlorine ion and make NaCl - salt. This union is called an ionic bond.
Chemists understand another way atoms can achieve electron stability in the valence shell. It is a system of sharing valence electrons with neighboring atoms and is called a covalent bond. For example, neutral hydrogen has only one electron, but needs 2 to fill its valence shell. Two hydrogen atoms can team up and share their electrons, forming a covalent bond. This is why hydrogen gas is a diatomic molecule, designated H2.
A battery is simply a way of storing electricity using chemistry (chemical potential energy). Some chemicals release electrons (oxidation) in a reaction and some chemicals absorb electrons (reduction) during a chemical reaction. Here is one example of how this is accomplished.
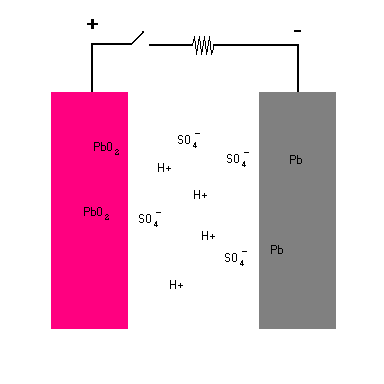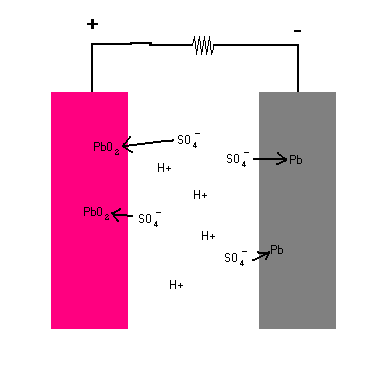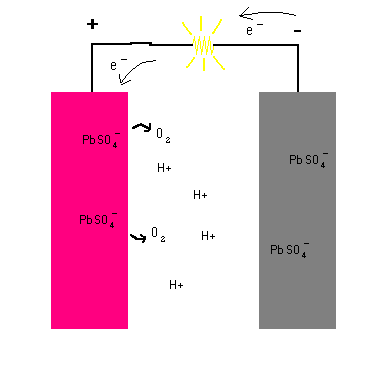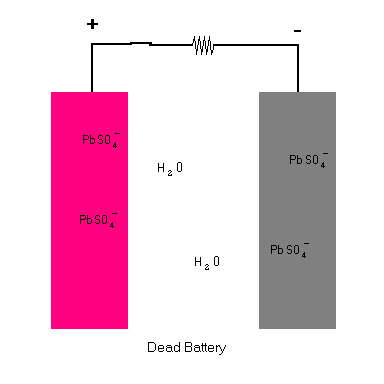
Your car battery consists of a number of isolated cells. Each cell has two plates ... an anode (the negative terminal) and a cathode (the positive terminal). The anode (negative) is lead (Pb) and the cathode (positive) is also lead with a coating of lead dioxide (PbO2). Both plates rest in a solution of water (H2O) and sulfuric acid (H2SO4) and is known as the electrolyte. The electrolyte serves as a medium through which charged particles can migrate between the two plates. The sulfuric acid in solution breaks down to form free flowing hydrogen ions (H+1) and sulfate ions (SO4-2).
When you connect the terminals of the battery together and complete the circuit, a chemical reaction takes place at each plate. Each step is animated in the diagram below.

Lead Storage Battery (animation)
Note: The potential difference between plates in this reaction is 2 volts. In order to produce the 12 volts most cars require, 6 separate cells need to be connected in series.
The key is seeing that when the circuit is completed, a chemical reaction takes place at each terminal ... one that releases electrons at one side and another that must take in electrons at the other terminal. The reaction keeps going until there are no more chemicals left to sustain the reactions. In the process, a potential difference (voltage) is established which compel free electrons to migrate from the anode to the cathode (and through the load). All chemical batteries use this basic principle whether it is car battery, batteries for a flashlight, watch, or calculator.
Keep in mind that there are many different kinds of batteries for many different applications. Your laptop computer, cell phone or iPod likely has a lithium-ion battery (link 3.2.a) (which has a very high energy density) and your cordless drill may have a nickel-cadmium (Ni-Cd) battery. All batteries have a voltage and current rating. Always dispose of batteries in an environmentally friendly way.
In the case of the lead storage battery, the chemical reactions at each plate were exergonic. That is, energy is released. The process of electroplating follows many of the same ideas, but the reactions at each plate are endergonic. That is, energy must be put into the system to complete the chemical reactions. Simply stated, if you replace the "load" with a battery, you can force chemical reactions to occur at each plate.
Electroplating is the process of coating an object with a thin layer of metal which comes from ions in solution.
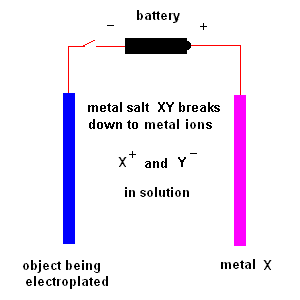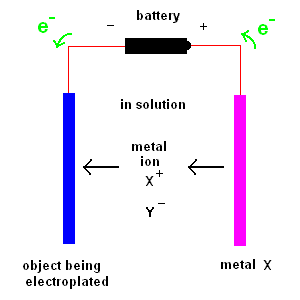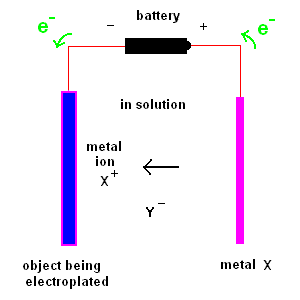
Two plates are suspended in solution. One plate is the object to be coated. The second plate consists of the metal you plan to coat (the first plate) with. The solution contains a dissolved metal salt (whose ions contain the "coat" metal). For example, if you wish to coat an object with nickel, you would immerse a nickel plate in a solution of NiCl2 - nickel chloride. A battery (or DC power supply) is connected as shown in the diagram above. The object being electroplated (shown on the left) receives electrons from the battery (acquiring a negative charge) which attracts the positive metal ions in solution. At the same time, electrons are removed from the metal plate (shown on the right) producing positive metals ions which are released to the solution (so the reaction continues).
One very promising way of producing electricity on a large scale is the fuel cell. A fuel cell is similar to a battery in that it can store electrical potential energy using chemicals. The chemicals in this case are hydrogen gas (H2) and oxygen gas (O2). When combined, they make ordinary water and release electrons which can be used to run a motor, power a computer, or light a lamp. Let's look at this closer:
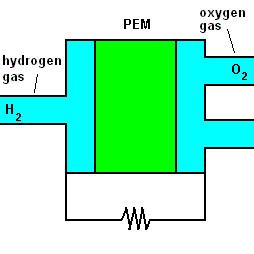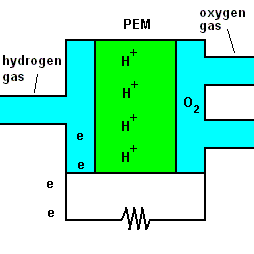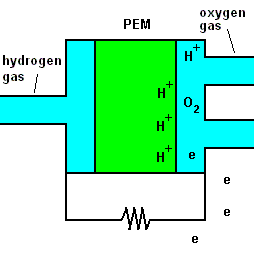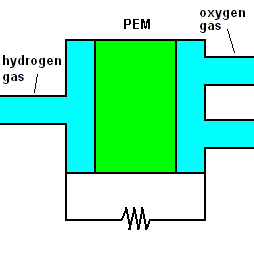
A fuel cell animation (animation)
The animation shows that once hydrogen gas enters the cell, it separates into positive hydrogen ions and electrons (with the aid of a chemical catalyst). The exact reaction at the anode (negative terminal) is: 2H2 => 4H+ + 4e-. A barrier known as a proton exchange membrane (PEM) allows only the positive hydrogen ions to pass. The electrons move through an electric circuit and combine with the hydrogen ions, along with some oxygen (which makes up 21% of the air). At the positive terminal (cathode), these particles combine to form ordinary water. The reaction is O2 + 4H+ + 4e- => 2H2O.
Why does this hold so much promise for the future? Because there are several ways we can obtain the raw materials - hydrogen gas and oxygen gas. One way is to reverse the process. During sunny days in the desert, a huge array of photovoltaic cells can be used to separate ordinary water into these gasses. This process is called electrolysis which is basically the opposite of a fuel cell. Another way is to refine existing hydrocarbon fuels (like methane or methanol) to make the necessary hydrogen gas using a device known as a reformer. Using fuel cells, we could significantly reduce our dependence on conventional fossil fuels and provide a cleaner fuel for the environment. The problem is developing a nationwide distribution system. So far, I've never seen one single hydrogen "gas station".
Alessandro Volta in 1800 made the first battery by placing dissimilar metals together. When he did, a small voltage was established between the metals. Chemists now realize that each metal has an "electrical potential" which is a measure of how easy or difficult it is to extract electrons from that substance.
Now consider the following arrangement:
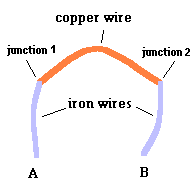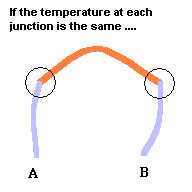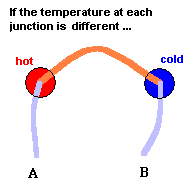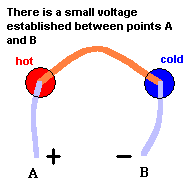
Key to understanding
a thermocouple (animation)
At each metal junction, a small voltage is established. Under normal conditions, the voltage between points A and B would be zero. This is because the electrostatic potential established at junction #1 would be cancelled out by the electrostatic potential established at junction #2 ... like taking two identical batteries and placing them so + touches +. But an interesting thing happens when you make one junction hotter than the other. The heat alters the "electrical potential" at the heated junction and a small net voltage is set up between points A and B ... like connecting the + side of a 3 volt battery to the + side of a 1.5 volt battery. This is known as the Seebeck effect if you care to investigate this phenomena further. The good news is we now have a device that can produce a net voltage (something needed to make electrons move) by exploiting differences in temperature. The bad news is, the voltage is rather low so this can only be used (economically) with devices that require very little power. Note: p=v2/r where p is power, v is voltage, and r is resistance. This device does, however, have its uses.
It was found that a thermocouple will run in reverse! That is, if a voltage is applied across points A and B, one of the junctions will heat up and the other will cool! This is known as the Peltier effect and a discussion of the physics involved is beyond the scope of this course. Have you ever seen a type of cooler which runs off your 12 volt car battery (via the cigarette lighter)? These are called thermoelectric coolers and they are commercially available now.
Are there other ways to make electricity? You bet ... but that is covered in another section. Stay tuned!
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author