Key Terms
Carnot engine
diesel engine
Newcomen steam pump
Otto cycle
two stroke engine
Watt steam engine
The Industrial Revolution was born when we learned to harness the power of steam. You already know that many great things can be accomplished when a wheel spins. The steam engine did this easily and cheaply thanks to inventors, Newcomen and Watt. Suddenly the ability to transport cargo and passengers took a quantum leap. Industry could pound, grind, lift, and pull by the power of steam. Factories sprung up overnight and cities brought in rural residents in droves. Early steam engines were powered by coal (and some by wood) at great cost to our environment. Although we have switched to petroleum for most of our transportation needs (internal combustion engine), we still use coal (and natural gas or nuclear energy) to make steam ... which drives huge turbines ... that generate our electricity. The environmental problems we encounter come from the choice of fuel we use to make steam, not the machinery. Several prototype power plants have found cleaner and "greener" ways of making steam to drive turbines. Steam is not going away anytime soon.
All heat engines look for ways to convert the potential energy of a high pressure gas into mechanical (kinetic) energy. Steam drives a turbine, but the internal combustion engine explodes a fuel to push a piston down a cylinder. This linear motion is converted to rotational motion of a crankshaft. How this is accomplished is covered in this section. In addition, innovative designs for alternative heat engines are explored.
Hero's Engine
The first concept of using heat to make things move was Hero's Engine (ancient Greek). It amounted to nothing more than entertainment as jets of steam made a sphere spin around.
In 1698, Thomas Savery found a way to "lift water with fire" with the Savery steam engine. The need was certainly there. Miners were always plagued with water in their way. The standard method for removing water out of mines was to use horses to lift buckets. Savery's system used the pressure of steam to lift water ... or better ... the lack of pressure. When steam condenses, it produces a sudden drop in pressure (vacuum). Savery allowed steam to condense in a chamber at the top of a column of water. This allowed atmospheric pressure (at the bottom of the column) to "push" water up a tube and into a reservoir (producing a 26-28 feet vertical lift) in the same way you use a straw to drink liquids. In addition, the water in the reservoir could be propelled another 80 feet higher by the positive pressure of steam. His model (built in 1699) was inefficient and subject to boiler explosions.
The first steam engines of Newcomen (1712) were very inefficient and only used to pump water out of mines. The idea was quite simple. Click link 5.1.a to see a nice animation.
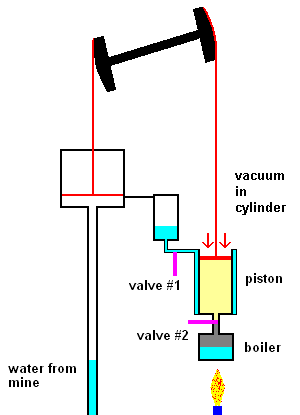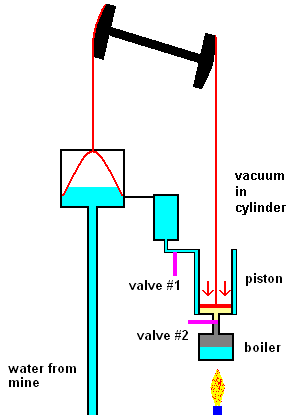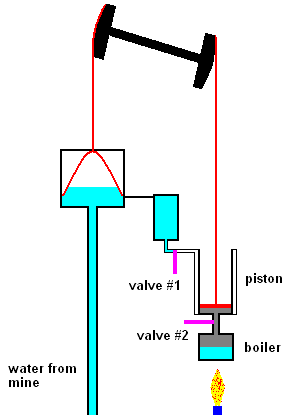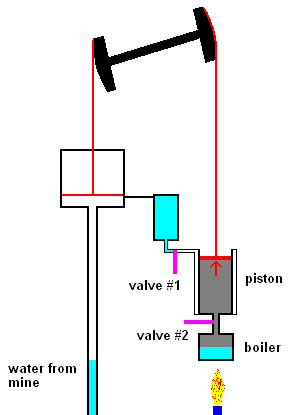
The Newcomen steam pump (animation)
The main points are:
Slight improvements were made in this system, but it was still rather inefficient. James Watt dramatically improved the design of the steam engine so it became a practical invention. Watt realized that it was a huge waste to use one vessel which needed to be heated up (to produce steam) and cooled down (to condense steam). His improvement used a separate chamber to produce the steam (always hot), then utilized valves to ensure that high pressure steam could always exert forces on a piston as the animation below illustrates.
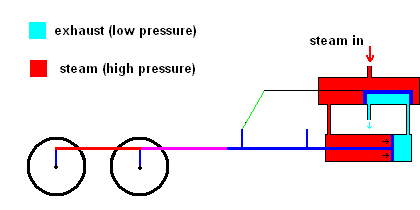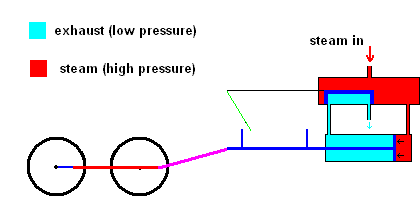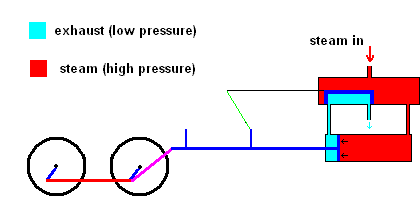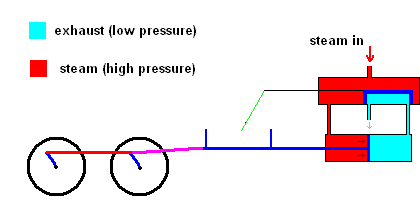
Watt steam engine (animation)
Vehicles which ran on steam were invented but short lived because they were heavy, slow, and impractical. Enter the age of petroleum and gasoline powered cars. Many inventors made early prototypes, but the first successful internal combustion engine was invented in 1876 by Nikolaus August Otto. His engine serves as the model most all vehicles on the road utilize today and the design is known as the "Otto cycle". In 1885 Gottlieb Daimler modified the design to include a vertical cylinder and carburetor to inject gasoline into the cylinder.
The basic idea is to use the explosive pressure when gasoline is ignited to push a piston down a cylinder. The gasoline is ignited by a spark plug which is designed to fire when the piston reaches a precise location. This delivers energy to the crankshaft. The animation below demonstrates the 4 strokes in the Otto cycle.
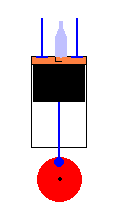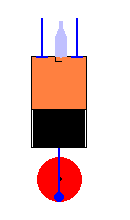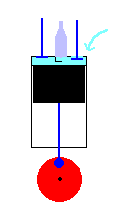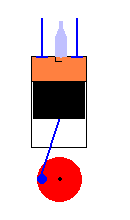
A 4 cycle engine in action (animation)
The engine is called 4 cycle (or 4 stroke) because the movement of the piston is associated with 4 distinct tasks that need to be performed in proper sequence. The four cycles are (in order) ... starting with the intake stroke:
Intake Stroke - The engine needs to prepare itself by drawing a gasoline/air mixture into the cylinder which enter the
chamber through an intake valve.
Compression Stroke - The piston compresses the gasoline /air mixture to a
high pressure (which gets even higher after ignition).
Power Stroke - The gasoline ignites the pressurized mixture of gasoline
and air. This exerts great force on the piston which drives it down the
cylinder (turning the crankshaft in the process).
Exhaust Stroke - When the piston moves back up, it removes exhaust gasses out
of the cylinder chamber by pushing them out an exhaust valve.
This animation at link 5.1.b does a great job explaining these steps.
Notice that a single piston only delivers power on one quarter of the cycles. To even the power distribution, cars have many pistons (usually 4, 6 or 8 cylinders). The system is engineered so the power strokes are staggered. That is, at any one time at least one piston is delivering power to the crankshaft.
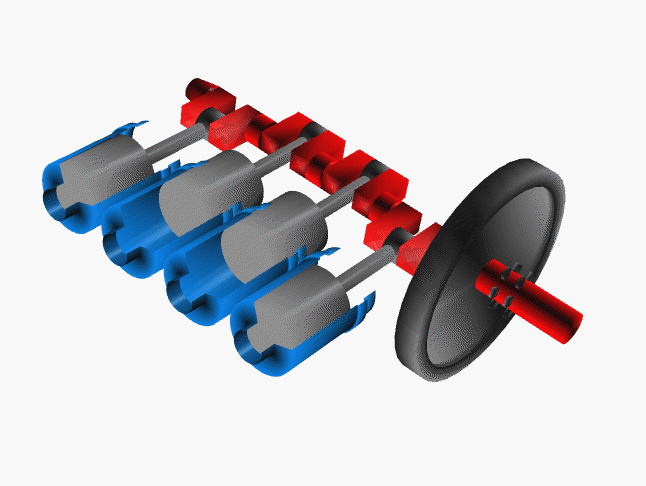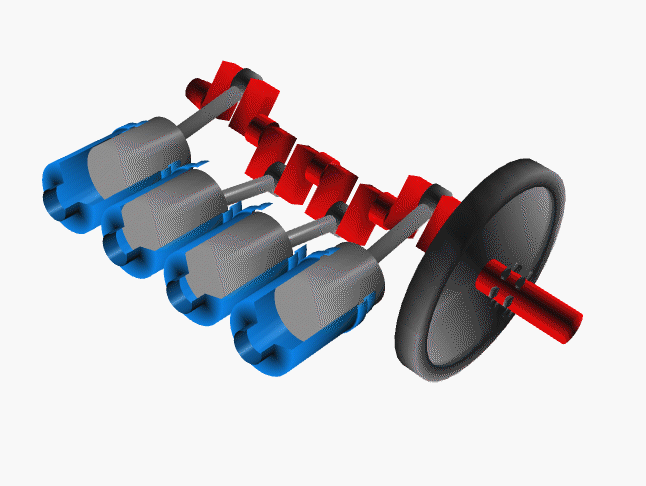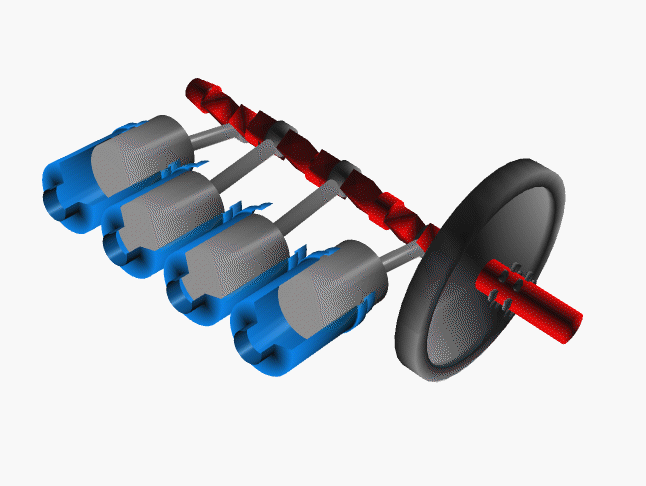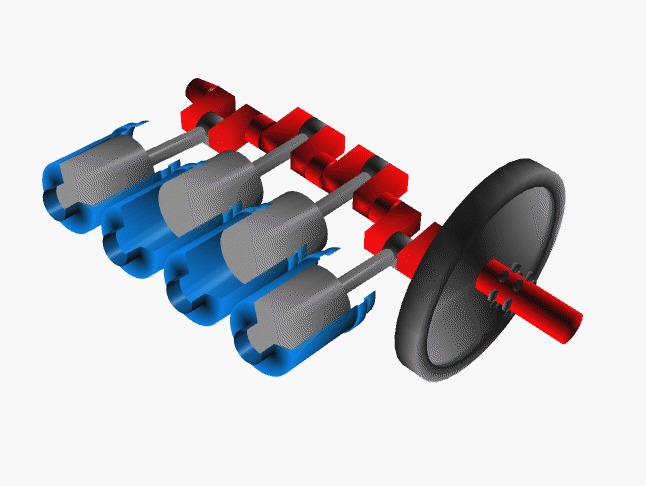
Credit NASA
Cylinders are pressure tight containers, so you need to find a way to inject fuel and portals to eject exhaust gasses. Valves act like gates which open and close ... permitting the aforementioned access. The valves are normally closed, forming a tight seal by spring compression. They are opened by a simple cam ... called the camshaft which works against the spring and lifts the valves from the seat. The motion of the camshaft is geared to spin once for every 2 times the crankshaft rotates. If not, valves would open during the power and compression stokes.
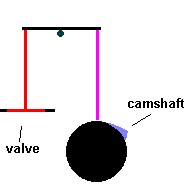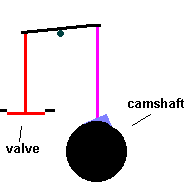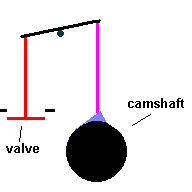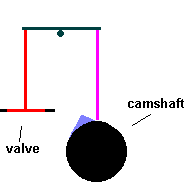
The Camshaft (animation)
If interested, click link 5.1.c to see a nice animation of valves in action.
To create a spark, a very high voltage is required (over 40,000 volts). This is the job for a step-up transformer. But if you recall when we discussed transformers ... the primary coil must receive AC ... and the electrical system in a car works off 12 volt DC. Big problem! If a steady direct current flows through the primary coil ... there is no changing magnetic field ... and no current at all is induced in the secondary coil.
To get around this problem, earlier cars had breaker points. Breaker points were electrical contacts that could be broken by the action of a cam. When the cam broke the electrical contact supplying the primary ... the magnetic field would rapidly collapse ... and it was this changing magnetic field that would induce high voltages in the secondary coil.
In modern cars, the timing is all done by microcontrollers. Pick-up sensors (Hall Sensor) tell the computer the exact position of the crankshaft. When the computer determines when a spark should occur, it opens a transistor, allowing a pulse of 12 volt DC current to the primary coil of a transformer. You should see why a pulse of DC current will work here.
If interested, read link 5.1.d which explains how a (4 cycle) car engine works.
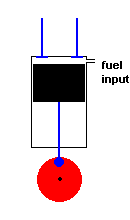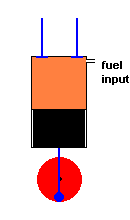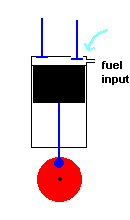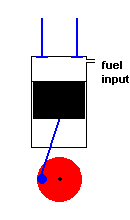
A diesel engine (animation)
Diesel engines work slightly differently from conventional engines. During the intake stroke, the engine only accepts air (not an air / fuel mixture). During the following compression stroke, only air is compressed and heated. Because the cylinder only contains air, there is no fear of any kind of pre-ignition (which could happen in a conventional engine if compression becomes too high). With this approach much greater compression ratios are possible, and this improves efficiency. In fact, diesel engines do not have spark plugs at all! This is because the fuel is injected into the cylinder at the height of air compression. The heat of the compressed air is sufficient to ignite the fuel, and this initiates the power stroke. After the exhaust stroke, the process is repeated.
Since diesel engines rely on the heat of compressed air to ignite the fuel, a cold engine in the morning could be a problem (and it does get cold during winter in Wisconsin). To solve this problem, diesel engines are equipped with "glow plugs" which pre-heat the cylinders by electrical resistance so it will start ... even on the coldest of mornings.
If interested, read link 5.1.f which explains how diesel engines work. Link 5.1.g shows a great demonstration.
Gas powered weed eaters and chain saws usually run off a 2 stroke engine. These engines require you to add oil to the gasoline ... and it produces a lot of pollution when running. This type of engine has only two cycles - power stroke and exhaust. This, in theory, makes it more efficient, but it also means you have to take some shortcuts to get that efficiency.
To make this system work, you have to make use of the space outside the cylinder wall. In the engines listed above, this space is normally filled with oil for lubrication. In a 2 stroke engine, this space is filled with an air/fuel/oil mixture. This is why you have to add oil to the gasoline. During the power stroke the piston is being forced downward and at the same time, compressing the air/fuel/oil mixture in this surrounding reservoir. When the piston is moving upward and exhausting the ignited gasses, the motion of the cylinder creates a negative pressure in this surrounding cavity. This allows air/fuel/oil to flow into the chamber for the next cycle. A 2 stroke engine has no valves (except for a check valve at the intake) because the motion of the piston covers/uncovers access portals.
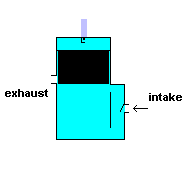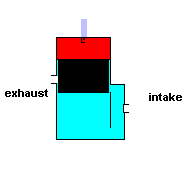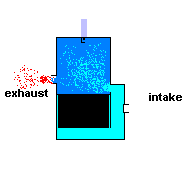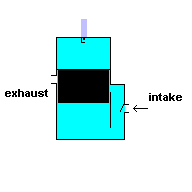
A 2 stroke engine (animation)
If interested, read link 5.1.g which explains how 2 stroke engines work or watch the animation at link 5.1.h
Please skim this over lightly.
Engineers working on early steam engines found many ways to improve the overall efficiency of the device. However, they were very much relying on trial and error techniques in their quest. They had no theoretical model to work from and the physics of heat was not well understood (thinking heat was fluid called caloric). Only well after heat engines had been around for quite some time was the physics (and theoretical limitations) of heat engines understood.
In a heat engine mechanical work is done on the piston by the action of an expanding gas. Recall the first law of thermodynamics ... energy is not created or destroyed ...it only changes form. At the start of the power stroke (after the fuel is ignited) the gas is at a very high temperature and pressure. As it pushes on the piston, it expands and cools to a lower temperature and then released to the atmosphere. It may be intuitively clear that the gas loses energy during this process, and this loss shows up as useful work. The first law of thermodynamics is safe here. However, the second law of thermodynamics may seem in jeopardy here.
Any discussion about the second law of thermodynamics is much more abstract. Most students have a difficult time grasping the concepts ... but we will try. When we first discussed the second law of thermodynamics, we showed that the natural tendency was from "ordered" kinetic energy to "disordered" random heat energy. But there are other ways to define "order" and "disorder". Physicists use the term entropy to measure the condition of disorder in a system. In fact, some books will state the second law of thermodynamics as: "In a closed system, entropy will always increase". This is a fancy way of saying that in a closed system, the tendency is toward a state of greater disorder. If heat is "disorder" and the motion of a piston is "order" ... aren't we violating that principle with a heat engine?????
Let's answer that with a simple analogy. Consider two identical objects resting together on a table top (case 1). In a second situation (case 2), the two objects are separated. One is lowered to the floor and the other is raised the same distance above the table. In both cases the total energy is the same ... but the difference is: In case 1 (resting together), one object cannot affect the other object. In the case 2, one object has the potential to interact with the other object (one could fall on the other and both could break).
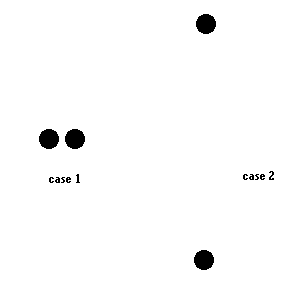
A physicist would see a difference in "order" between these two situations. Each would have the same total energy ... but in case 2 there is a potential to do something not possible in case 1. Case 2 has more "order" than case 1 (or case one has the higher entropy).
Now consider a similar situation involving gasses. In case 1 (below) a great mass of gas is all at the same temperature. In case 2, there are two separate masses of gas ... one with a higher temperature than case 1 and the other with a lower temperature than case 1 (so that the total energy of both cases are the same).
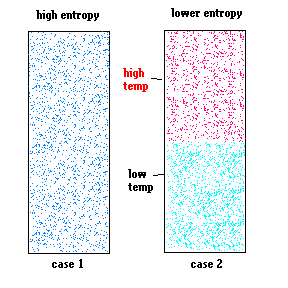
Although both containers contain the same amount of thermal energy, case 2 has a higher state of "order" (lower entropy) than case 1 because there will be a net flow of heat between the gasses that will not occur in case 1 (heat will flow from high temperature to lower temperature). When it does, there will not be a change in the total energy of the system ... but there will be an increase in the system's entropy.
Don't worry if all this does not sink in at the first reading. As a student I didn't "get it" either on the first take. It was only after I was introduced to another example did it all start clicking in my brain. I saw a demonstration of a water wheel driving a water pump. In the demonstration, I saw the action of the pump push water above the reservoir of the water wheel. My initial reaction was that this was violating everything I understood about energy. How can you pump water to greater heights if you are using the energy of the water to propel it in the first place??? The answer came when I was shown that an extremely large amount of water was losing energy as it flowed over the water wheel .. but only a tiny amount of water was gaining energy. It was a trick ... a very clever trick to make me understand that much more gravitational potential energy was being lost then was gained .... overall. It started giving me insight to what entropy was all about. The neat thing here is that a heat engine is playing the same trick here.
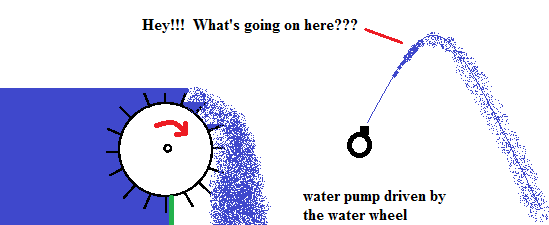
A heat engine will take advantage of this heat flow and try to convert some of the heat transfer to work (ordered energy).
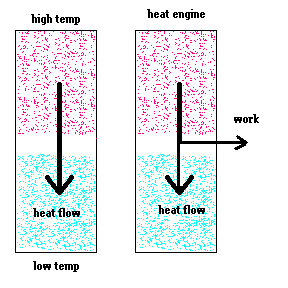
The reason a heat engine does not violate the second law of thermodynamics is because:
This is a very fancy way of saying that all heat engines work under certain
restrictions. Here are a few statements dealing with
heat engines and the second law of thermodynamics that you can understand:
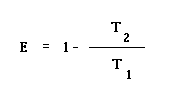
where
E = efficiency
T2 = temperature of the cold region (measured in Kelvins)
T1 = temperature of the hot region (measured in Kelvins)
In 1824 Sadi Carnot laid down the theoretical framework for heat
engines. In his book, Reflections On The Motive Power Of Fire, he
describes the operation of "ideal" heat engines. Many of the concepts in
the preceding section came from this brilliant thinker. Carnot was way
ahead of his time and his insight led to the development of the Second Law of
Thermodynamics.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author