Key Terms
ballast
Bohr model
energy levels
fluorescent lights
phosphorescence
photon
spontaneous emission
Vapor lamps produce light when electricity (moving electrons) passes through a gas under low pressure. It does not rely on "heat" to make light, ... it uses the atomic properties of the individual atoms to produce light. Here is some science you need to understand:
You have been taught from a young age that an atom consists of a nucleus (made up of protons and neutrons) and the electrons orbit this nucleus. This is true, however, the rules of quantum mechanics make the story a bit more complicated. Here are some additional things you may not have already learned:
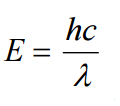 where
h is known as Planck's constant and c is the speed of light.
Notice that as wavelength decreases, photon energy increases.
where
h is known as Planck's constant and c is the speed of light.
Notice that as wavelength decreases, photon energy increases.Niels Bohr solved this mystery of light and the atom. We will use his hydrogen model to illustrate the idea since hydrogen is the simplest atom (one proton and one electron). Below is an animation which depicts a crude representation of the energy levels the single electron is allowed to orbit in (black circles). If left undisturbed, the electron orbits in the ground state ... the closest it can get to the nucleus. If the atom absorbs energy from its environment, the electron moves to a higher orbit. When it drops back down, the atom emits a photon of radiation.
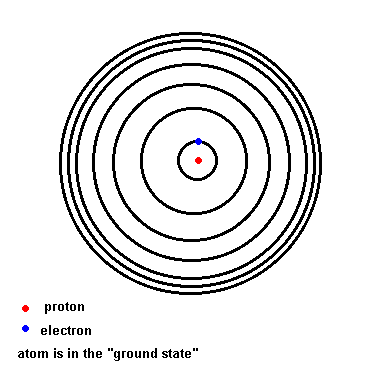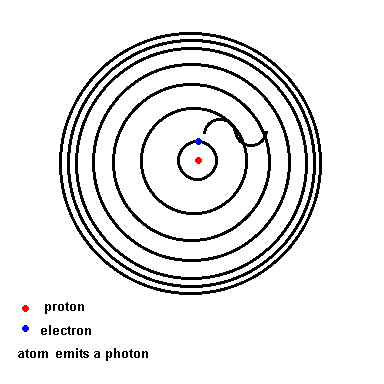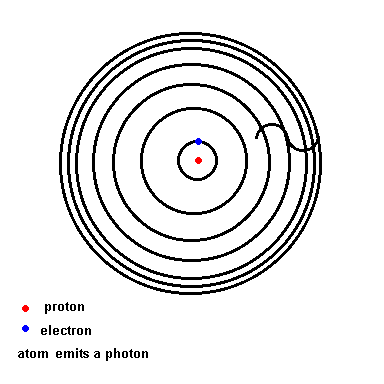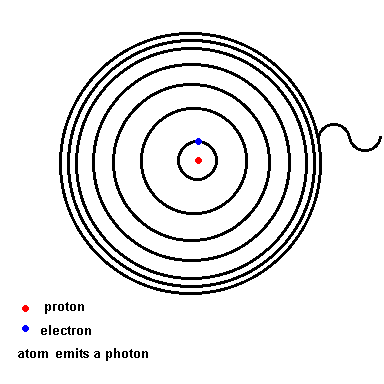
The Hydrogen model (animation)
The energy levels are not uniform, like steps on a staircase. Instead, they become progressively closer the farther they appear from the nucleus. The transition from orbit 2 down to orbit 1 (ground state) is such a large energy transition that the photon released lies in the ultraviolet section of the electromagnetic spectrum. There are only 3 downward transitions in the hydrogen atom that produce light visible to our eyes. These are depicted in the diagram below.

Why we see 3 colors in the spectrum of hydrogen.
![]()
A "bright line" spectrum of hydrogen.
To see this you would need to fill a tube with low pressure hydrogen gas and zap it with a source of high voltage electricity. The electrons moving through the gas transfer some of their energy to the hydrogen atoms. The atoms would then release this energy. Since this is happening to millions of atoms at the same time, the net effect is an eerie orange looking color. The next step is to pass this light through a prism to reveal the separate red, blue-green, and violet light coming from the gas.
If interested, click link 5.5.a to see a Java applet ... notice that the "bluer" the light ... the bigger the electron jump.
Mercury vapor lamps are also known as fluorescent lights. Although there are several different types of fluorescent light designs, we will just cover the typical one you may have in your home or office - the hot cathode type.

Inside each tube is an inert gas (typically argon) and a tiny bit of mercury. At each end of the tube are two electrical connections which (on the inside) look very much like the filament found in an incandescent light bulb. However, the function of these filaments (called tungsten electrodes) is not to give off light, but rather, electrons ... which boil off the surface when heated. Driven by voltages established at each end of the tube, these free electrons are compelled to move across the tube which interacts with the mercury vapor .... exciting those atoms as electrons are driven to higher orbits. The mercury atoms then spontaneously release that energy in the form of photons in the UV portion of the spectrum. These UV photons must pass through a phosphor coating on the inside of the bulb, which absorbs this energy and releases it in the form of many photons all within the visible portion of the spectrum. The combined effect is white light.
First, the free electrons that boil off the tungsten electrodes and start moving will interact with the argon gas and ionize it. This, in turn, create the conditions found in lightning ... a discharge arc. This is necessary because the mercury within the tube is in the liquid state and the discharge generates energy to turn the mercury into a vapor. There are several ways to kick start this process and most involve a much higher start-up voltage. Once an arc is established the voltage can drop to a lower level. Since this current is driven by AC voltages, the current is constantly flipping direction.
.... but we don't want too much current. Enter the ballast.
Electrons moving through a vapor behave a bit different from electrons moving through a conducting solid. In solids, the electrical resistance remains fairly constant. However, in a vapor lamp things are more complicated. To establish a conducting path when the fluorescent light is first turned on, heating elements emit electrons which quickly ionize some of the vapor. As stated earlier, this is needed to establish a conducting path. However, once established, the electrons start moving across the vapor, and they tend to ionize even more gas atoms. As a result, the overall electrical resistance drops and, left unchecked, the amperage would jump to unsafe levels (as in a bolt of lightning). You need a way to limit the current and that is what the ballast does.
One type of ballast (called a magnetic ballast) is simply a wire wrapped around a metal bar. AC currents run through this device before running through the vapor lamp. This particular type of ballast is no longer used because someone invented a better ballast in the form of an electronic ballast. However, we can still discuss the magnetic ballast since it is fairly straight forward.
A magnetic ballast is just the addition of an inductor in the circuit. If you recall from an earlier section (unit 3 - induction), an inductor is really nothing more than a wire wrapped around a metal bar. In this particular application, as AC current moves through the system, the "eddy currents" produced in the bar generate their own changing magnetic field ... which then induce a "back emf" or back current in the coil (remember the metal detector?). The direction of this "back current" in the coil is in opposition to the current that first started all the action. This tends to act like a "current brake" in the system and prevents the vapor lamp from going into this "run away" condition we first mentioned. It makes sense that old timers call the ballast a "choke" ... because it chokes the current. All vapor lamps require a ballast to run safely.
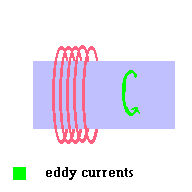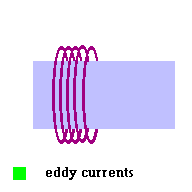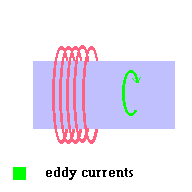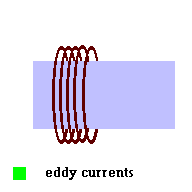
The magnetic ballast
Actually the ballast does more than just limit the current. It also provides a boost voltage when the lamp is first turned on (to generate the discharge arc) as well as preheat the tungsten electrodes. No ballast ... no vapor lamp.
Click link 5.5.b for a video
Aurora (Northern Lights) are a result of electricity from a solar flare interacting with the earth's magnetic field and making the gasses in our atmosphere glow. The colors in the picture below are produced when electrons in nitrogen and oxygen atoms are excited to higher orbits by incoming solar particles, then drop to lower orbits (giving off the light you see).

Aurora
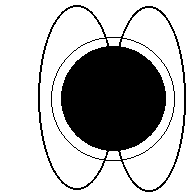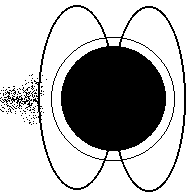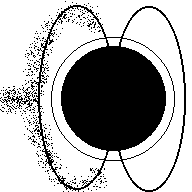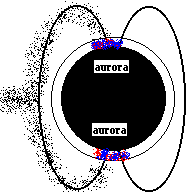
Charged particles from the sun (animation)
Nebulae Giant clouds of gases in space are called nebulae. These nebulae produce light in the same way a vapor lamp emits light. That is, the gases produce bright lines in the spectra. Usually hot objects (stars) buried deep in the nebulae provide the energy to excite the atoms in the gas. Astronomers study the light from these gas clouds to discover what elements they are made of. Each element produces a unique pattern of lines. By studying these spectra, astronomers know that when a massive star goes supernova (blows up), they also "manufacture" and disperse many elements found on the periodic table. Check out these spectacular pictures.The Crab Nebula
link 5.5.c
The Helix Nebula
link 5.5.d
The Orion Nebula
link 5.5.e
Metal Halide Lamps - These are high intensity vapor lamps filled with argon or high pressure xenon (the expensive headlights on cars that look blue ... known as xenon headlights). These are also known as HID (high intensity discharge) lights.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author