How Nature Makes Mineral Deposits.
Nature concentrates minerals all sorts of ways. Here are just
a few.
How to form minerals with water.
Water always has minerals dissolved in it. Have you ever examined
an iron or vaporizer once the water has boiled out of it? If not,
take a pan of water and boil all the water away. A thin film of mineral
deposits are caked on the pan surface. We buy water softeners to
remove most of these minerals but it is actually the minerals, which gives
water its flavor.
Minerals formed by Evaporation and Precipitation
-
Now imagine you had some sea water (perhaps a large section of ocean gets
land locked....like Lake Bonneville in Utah was long ago). As the
water evaporates, the remaining water gets saltier and saltier (The Great
Salt Lake). Eventually, solid salt gets precipitated out of the solution
and gets deposited. The Salt Flats in Utah are a fine example of
this. These are sometimes called brine or evaporate
deposits.
-
Gypsum, an important industrial material used in home construction (wall
board), is formed in this way.
-
This same thing happens in caves. As water (percolating through the
ground) reaches air in a cave, some of the carbon dioxide (CO2)
escapes from the drop ... leaving behind calcite. This is how a stalactite
is formed.
-
Banded Iron Formations were formed 1.8 to 2.2 billion years ago
when oxygen started building up in the atmosphere. The oxygen dissolved
in the ocean water and reacted chemically with iron ions in solution ...
forming solids which settled as thick deposits of iron oxide.
-
Likewise, thick deposits of limestone (calcium carbonate) formed (and are
still forming) when sea creatures (coral, snails, clams, shells, etc.) used
carbon dioxide molecules and calcium ions in sea water to construct
armor. Their skeletons settled to the sea floor forming thick layers
of limestone.
Hydrothermal Solutions
Other ways to concentrate minerals
Igneous activity
Many minerals are concentrated as a result of igneous activity (melting
of rock). This might sound lame, but all you need to do is have magma
cool and it becomes a rock. So how does nature do this? You
might think of volcanoes first and you wouldn't be wrong. The Hawaiian
Islands are made directly from volcanic activity. But igneous rocks
can also be formed deep underground (intrusive).
There are many factors involved in the formation of a mineral directly
from a melt. Probably the biggest factor is the initial chemical
makeup of the magma. This can vary greatly from place to place (as you
will see later on in this class). Also, each mineral in the melt
has different physical and chemical properties that effect the outcome.
Finally, other factors such as the presence of water and the type minerals
surrounding the melt may be a factor. Let's just look at some hypothetical
examples.
Example #1 - Magmatic Segregation
Consider a chamber of magma deep underground. Geologist call this
a pluton or a batholith. Suppose it contains many different silicates in
its mixture. Nature has a way of sorting the different minerals as
the magma slowly cools. As you recall, the different silicates have
different melting (freezing) points. So as the chamber cools, and
the temperature drops, the first silicates to crystallize would be the
ones with the highest melting (freezing) temperature. This would
be silicates from the olivine group. Once solid, the density would
increase and these crystals would sink to the bottom of the chamber.
This process would then continue to the pyroxene group....ending with quartz
(with the lowest melting point). The final result is a stratified
layer of separated minerals....olivine at the bottom and quartz at the top.
Rich nickel and copper sulfide ores were formed in this way in Sudbury,
Ontario.
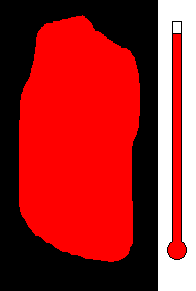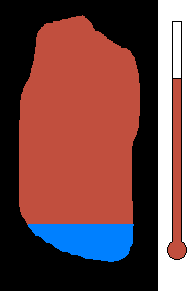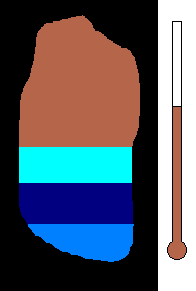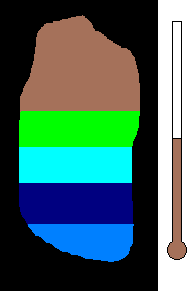 (animation)
(animation)
Example #2 - Contact Metamorphism
... or "rock chemistry" occurs when solid rocks are in direct contact with
liquid rock (magma). Often the reactions are quite complex and sometimes
not well understood, but it does account for several deposits of
minerals.
Suppose a large deposit of limestone is intruded by a vast volume of
magma deep underground. In the presence of water, calcium atoms (in
the limestone) can be replaced by iron atoms (in solution). This is just
one example where atoms of one type are "replaced" by atoms of a similar type
(size and charge) to produce a new mineral. In similar reactions valuable deposits containing tungsten and tin
may result.
Example #3 - Regional Metamorphism
Some minerals are produced (from other minerals) as a result of exposure
to extreme heat and pressure. Minerals such as asbestos, talc, and
gems like ruby and sapphire are found at the roots of mountain belts.
Example #4 - Diamonds are formed at great depths below the surface (a
metamorphic process). On occasion, magma will flow towards the surface
in a "pipe" through a region containing diamond crystals. These diamonds
are "scooped up" in the magma and transported toward the surface.
Diamond "pipes" are found in South Africa, Colorado, Wyoming and there
is even one in Arkansas (where I tried to "strike it rich" one hot day a
few years back in the
Crater
of Diamonds State Park).
Example #5 - Sometimes gasses escape from a melt (or solution). When
this happen, the melt (or solution) may lose its ability to hold minerals.
The vapors escaping from a melt carry these minerals in a gaseous state where
they may precipitate on a nearby surface ... forming some beautiful crystals.
Example #6 - Partial Melting
Why are the continents mostly granite? Good question and one which
you had better know the answer!
Plate tectonics is constantly pushing plates together and pulling them
apart at different parts of the globe. Much of this will be covered later
in this course. For now, consider two plates in a head on collision - one
consisting of oceanic crust (very thin and composed of basalt) and the
other made of continental crust (a bit thicker and mostly granite). Upon collision,
which is likely to forced down (under the other)???? Can you make
an educated guess? Scroll down for the answer (after you guess).
The answer is: Oceanic crust will always be forced under continental
crust! Why? Because basalt is denser than granite!
Once forced down, it will start heating up!
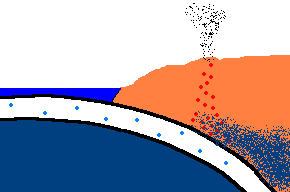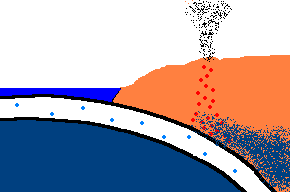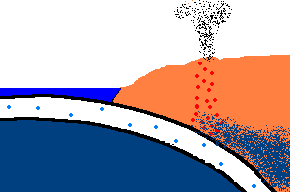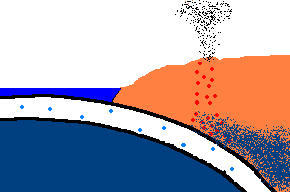 (animation)
(animation)
As the oceanic plate subducts under the continental plate, the temperature rises and the solid rock of the oceanic
crust will start to melt. Remember, this is oceanic crust and has a rather
"silica poor" content ..., but it is not zero by any means and does contain
additional silica from rocks washed to the sea from rivers and streams, as well.
So what is the first (and maybe only thing) to melt? The answer is:
the minerals with the lowest melting point ... the "silica rich" minerals
(quartz, feldspar, mica)! And even though there may not be much available
in this sinking plate ... we just found a way to separate the small amounts of
"silica rich" minerals from the subducting oceanic plate. This is called
partial melting. There is even more to this story. This subducting
crust also carries
with it water ... and water has the magical property that it can lower
the melting point of the surrounding upper mantle rocks. Actually these
surrounding rocks only partially melt because it only gets hot enough to
liquefy the lower melting point, "silica rich" rocks. This liquid
rock becomes more buoyant and starts to rise to the surface. OK .. it gets
even stranger!!! This molten rock undergoes additional changes in composition on its way up
to the surface. As it cools, it crystallizes
out any remaining "silica poor" basaltic type rocks (because they solidify at a higher temperature),
leaving the remaining (rising) magma even more silica rich. But what it this? ... the
stuff you make granite out of ... and granite makes continents! OK
- got that? Please re-read this last paragraph several times until you get
a clear picture how this process works.
Will this liquid (silica rich) magma make it to the surface????
The answer is: Not likely (although it can and sometime does in the form of a
rock called andesite). Why?
There are several reasons why this magma will most likely never make it to
the surface. One deals with viscosity. As you recall, this melt is
rich in silica and this makes it run like honey....very slowly. Another
factor is the temperature ... as this magma rises, it cools and quickly reaches
the freezing point. It becomes a block of granite at the base of the
continental margin. But do you see what this means? We've just
filtered out the small amounts of "silica rich" minerals (quartz, feldspar, mica) from oceanic
crust (and surrounding upper mantle) and added them to the edge of a continental
margin. And it will keep doing this as long as the oceanic crust
gets subducted under the continental crust. Pretty neat...Yes?
This is the process which keeps making continental crust and why this
continental crust
is mostly granite.
But you might be thinking now, "Does this mean there is more and more
land (continental crust) on the earth as time goes on"? The answer is NO.
The percent of continental crust (vs. oceanic crust) has remained
fairly constant for quite some time now.
There are other things happening at the same time....and these will
be covered in another class.
Click here to get back to the study guide.
ŠJim Mihal 2004, 2006 - all rights reserved

 (animation)
(animation) (animation)
(animation)