Answers to discussion topic questions in Unit 2
Please make sure you understand the answers before you attempt the practice
quiz or unit test. Many of these answers came from your fellow students.
Rotation
- Plasma and platelet donors have blood extracted and it is separated into
its component parts by a centrifuge. The target material is
isolated and the remaining components are transferred back to the donor. A
centrifuge is a device that spins the blood in a circle. Explain how this is
accomplished considering that ALL the components of the blood are trying to
"flee" the center in this device.
It all comes down to density. If you place water
and oil in a container, the oil always moves to the top because of density
(regardless of the amount or volume involved). In a centrifuge, the same
rule applies. The liquid with the highest density will move farthest
from the point of rotation. Density is just mass per volume ... a
measure of how compact the substance is. BTW, red blood cells have
a higher density than blood
plasma.
- A mass spectrometer is used to identify the chemical
composition of a substance. In what way is this discussion on rotation
pertinent to the way this machine functions? What force provides the
centripetal acceleration? How is molecule "A" distinguished from molecule "B"
if both ions have the same electric charge?
The first step is to give the atoms a positive charge by
stripping off electrons (which have almost no mass). These positive ions
are accelerated and then move through a magnetic field. Charged
particles are deflected in an external magnetic field because any moving
charged particle produces a magnetic field of its own. The two fields
interact and the particle is deflected by magnetic pushes. The amount of
deflection depends on the speed of the particle (which is known), the charge
of the particle, and the mass of the particle. For simplicity, if we
only consider ions with a +1 charge and all ions are given the same speed, the
amount of deflection then only depends on the mass of the ion. As an
analogy, if cars are moving along the highway at 60 MPH and subject to
collision from the side, the more massive trucks would deviate the least from
their path and the lower mass passenger cars would show the most deflection.
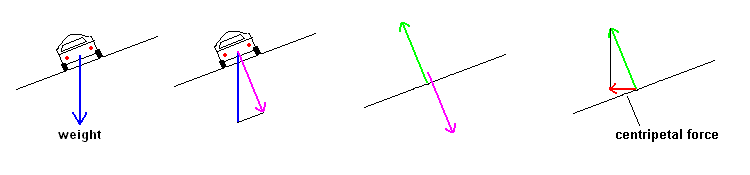
- What provides the centripetal force when your car turns on an un-banked
curve? Why do speedways "bank" their curves? Explain in terms of forces.
If the road is flat, friction between the tire and road
provide the necessary centripetal force. However, you don't need any
friction if the road is properly banked. Using vectors, the
image shows that when the road is banked, part of the cars weight is directed
perpendicular to the road surface (shown in pink and called the "normal"
force. Since forces always act in pairs, the road pushes with an equal
and opposite force on the car (arrow shown in green). A component of
this force is directed toward the center of the circle. This is the
centripetal force shown in red. The steeper the bank, the greater this
force becomes. At any given speed, there is a "banking angle" that will
provide 100% of the centripetal force. That is, you could make the turn
even if the road was glare ice.
- Perhaps conventional type rockets will no longer be needed to transport
cargo into space. Experts have proposed a space elevator
could do the same job at a fraction of the cost. Explain how that is
possible and how it would work.
Have you ever tied a rope to an object and swung it
around your head? Now imagine a spider crawling along the rope as it
whirls in circles. The key to the space elevator works a bit like this.
One end of the cable is attached to the earth's surface (at the equator) and a "weight" lies just beyond the geosynchronous orbit (the center of the
mass of the entire system must lie at the geostationary point).
Tension is maintained in the cable as gravity from the earth pulls on the weight
(centripetal force) and the
weight pulls back on the earth (remember forces always act in pairs). The payload climbs the
cable until it reaches the desired height. The problem is finding a
super strong, lightweight cable. Carbon nanotubes may be the answer but
no one has perfected a large scale manufacturing process yet.
-
Why do space rockets launched in the US fly toward the
Atlantic Ocean rather than the Pacific Ocean?
The space rocket must achieve a
horizontal speed of about 18,000 mph to achieve orbit. Why not let the
earth provide some of that speed? The earth rotates from west to east so
a rocket resting on the launch pad already has some horizontal speed. At
the equator, this is about 1,000 mph. That number becomes less as you
move to higher latitudes. However, any speed you already have at launch
time is free if you move in the same direction that the earth rotates.
One student added that launching from Florida makes sense for two reasons -
(1) flying over the ocean offers a safer path (we all know things can go wrong) and (2) the
latitude of Florida is the closest we can get to the equator ... and thus, the
highest west-east linear speed. I'm guessing that launching from Hawaii
would be cost prohibitive.
- Find an instance where the "ice skater effect" is observed or put to use.
Did you ever notice that when someone is playing tether
ball they tend to swing at the ball late after it has gone around the pole
once or twice? This is like the skater effect. The ball gets moving faster
when the rope distance between the ball and the pole gets shorter. The player
is anticipating a speed consistent with the last hit. The speed actually is
greater due to the rope wrapping around the pole and this causes some players
to miss the ball.
Astronomers see examples of the "ice skater effect" often. The classic
example is seen in pulsars. A pulsar is the collapsed core of a dead
star but spins at incredible rates (thousands of revolutions per second).
Another example is seen in the earth-moon system. Tides are slowly
reducing the earth's rate of rotation (days are getting longer by .0023
seconds per century) due to friction. To compensate, the moon moves
further away from the earth (at 3.8 centimeters a year) to conserve angular
momentum in the system.
- Give an example of positive feedback (not already covered
in the eBook).
Answers may very but the feedback must accelerate things
to go even more off balance. Example: In a growing economy, the
more money I make, the more I can invest, The more I invest, the more money I
make.
- Give an example of negative feedback (not already covered
in the eBook).
Answers may very but the feedback must change things to
bring balance in the system. Example: If the population of a
species grows too big, there becomes less food available to feed the
population. The population drops due to starvation.
The Centrifuge
The centrifuge was first used to separate milk from
cream. It is now used and applied to:
- remove most of the water from your cloths when your
washer goes through the "spin cycle".
- remove solid sludge in water treatment plants (much like the
spin cycle listed above).
- enrich uranium 235 (for nuclear power plants) from
the more abundant isotope, uranium 238. (Note: Using porous
membranes is another technique used in the US).
- purification of Biodiesel fuels (to separate it from
water and Glycerin). It is also used to purify other types of petroleum
based fuels (like jet fuel).
- cleanup of the oil spill in the Gulf was aided by a
famous
actor's centrifuge
- separate components of blood (red blood cells,
platelets, plasma).
- used to isolate macromolecules (like DNA) from a
mixture of cellular debris.
- make wine by removing solids that would normally
form sediments and also remove yeast cells (in another stage of the process).
- simulate "g forces" on pilots or create "artificial
gravity" in a rotating space station.
- simulate high stress effects on structures (like
during earthquakes) to test the structural integrity.
- separate molasses from sugar syrup in a sugar
centrifuge.
- choose the sex of your offspring because the X
chromosome has a slightly higher density than the Y chromosome.
- dry your lettuce with a
salad spinner.
- Believe it or not, George and Charlotte Blonsky
invented
a device that spins a woman to aid in childbirth. I'll bet it was
George's idea.
Thermal Properties of Matter
- If a nut on a bolt (or jar lid) is difficult to move, should you apply
heat or cold to help loosen it? Hint: You know what the bolt will do if it
gets warmer or cooler. What happens to the inside diameter of the nut if it
gets hot? Will it increase or decrease?
Apply heat! Everything expands .. including any
tiny gaps that may exist between the nut and the bolt. Note: This
effect can be accelerated if you heat the nut quickly (with a torch) .... and
apply torque before heat is conducted to the bolt. This way the inside
diameter of the nut increases and the bolt's outside diameter is unchanged
(making the gap even bigger).
 animation
animation
Let's go one step further. How about when opening a stubborn glass jar
of pickles? Glass and metal both expand when heated but
metals expand more than glass when warmed to the same temperature.
This is similar to the thermal properties pointed out in the bi-metallic
strips (in this section). Because the metal expands more than the glass,
the small gap between these surfaces widens.
Note: I once worked at a facility that utilized the thermal properties of
materials. Before placing gears on shafts, the gear was heated and the
shaft cooled so it would slide on easily. The heat increased the
inside diameter of the gear and the cold shrunk the outside diameter of the shaft.
- Is the thermostat in your car designed to prevent the car from becoming
too hot or too cold? What is the reasoning behind your answer?
The common answer is that the thermostat is used to
maintain a constant temperature of the engine. I'll buy this answer.
However, what would happen if you had no thermostat at all? Your car would
run too cool because nothing would restrict the flow of coolant. Some might
ask ... wouldn't a cooler engine be better for wear and tear? Maybe, but you
need a hotter engine to maximize your burning efficiency (plus your heater
wouldn't work very well). If you think of it
that way, a thermostat is designed to prevent your engine from being too cool.
After the thermostat opens (at about 190 degrees) it can do nothing should the
temperature get even higher (water pump fails, etc).
- Rocks have been known to explode during a flash fire (I actually witnessed
this once). How is this possible? Hint: Why do ice cubes crack when you drop
them in room temperature water?
Heat causes expansion. When you drop an ice cube
into a drink, it cracks because the outer layers of the cube suddenly heat up
but the inner sections of the cube remain at the same temperature (it takes
some time for heat to conduct to the center of the cube). This produces
internal stress within the ice ... enough to crack the ice. You will
find (in a later unit) how this phenomena can be put to good use.
However, another common answer to this question is also acceptable. The
rock may contain pores filled with water. When the rock heats, the
pressure of the trapped steam may be enough to blow the rock to pieces.
- Why is the bi-metallic circuit breaker shown in the material NOT used in
most applications (such as your circuit board in your home)?
One student wrote: The bi-metallic circuit breakers have
some disadvantages. Because they are heat sensing devices they can be
adversely affected by changes in surrounding temperature. When operating in a
cold environment they will trip at a higher current level increasing the risk
of damage of some appliances. When operating in a hot environment, they will
trip in lower current levels resulting in unwanted equipment shut down.
Another student wrote: This type of circuit breaker
(as shown) would shut off when the current heats up the bi-metallic strip.
Once the strip cools a few seconds, and after it has opened, it will then
close again and complete the circuit. Without some other mechanism keeping the
circuit open once it is tripped, this would be a dangerous form of circuit
breaker for your home. This is, however, how many Christmas tree lights
or the turn signals in your car are caused to blink.
I added:
Cool .. I'll buy this but I was really looking for
the fact that the circuit has to first have dangerously high currents already
flowing before it trips. It all comes down to response time. Things could be over heating (as well) down the
wires before it trips. You need a quicker way of sensing a
dangerous overload and turning off the juice. We cover a couple of ways
in the next unit. I like your answer though.
- You can tell when your Thanksgiving turkey is done with a small device
known as a pop-up timer. When it pops up, time to eat. How does
this device work and is it reusable?
Inside this probe is a substance that melts at 185
degrees Fahrenheit to let you know the turkey is done. The "pop up"
section of this device is embedded (frozen) in this substance and once the
correct temperature is reached, it is free to move. A compressed spring
delivers the energy to push the probe out. To reuse it, just place the
probe in boiling water (where the substance once again melts), and push the
popup back in as it cools to room temperature. This locks the popup back
into the substance and re-compresses the spring as well. All set for the
next Thanksgiving feast.
- Why does metal always feel colder than wood, even though both are
initially at the same room temperature?
Metal is a good conductor of heat and wood is not.
The sensation of cold occurs when heat leaves your body. In this case,
heat easily escapes your body when you touch metal and not so much when you
touch wood. Thermal conductivity is important where you need to remove
excess heat from an area. The CPU of your computer generates a lot of
heat. Manufacturers apply a thin coat of thermal paste (goop that has a
high thermal conductivity) to the back of the CPU to help move heat to nearby
cooling fins (and then use a fan to blow it away .... that's convection).
- Suppose equal masses of dirt and water (at the same temperature) each
absorb the same amount of heat. Will both items reach the same final
temperature? Explain.
On a warm sunny summer day in Milwaukee, the weather
person will report "cooler near the lake". There is a reason for this.
When equal masses of water and soil each absorb equal amounts of heat, the
soil temperature will rise about five times higher than the water. On a
sunny summer Milwaukee day, the land will get very hot and the water
temperature will only rise a little. There is a big difference between
the specific heat of soil vs. water. Water has a high
specific heat, which simply means it takes a lot more heat to get water
hot. Keep that in mind when you bite into
hot pizza. The hot cheese has a high specific heat, so a large
quantity of heat is transferred to the roof of your mouth when the two
surfaces meet.
Applications in Cryogenics - Cryogenics deals with the behavior of
matter at very low temperatures. Your mission is to post one important
and/or practical application in the field of cryogenics. You do NOT have
to discuss how these low temperatures are achieved.
One practical application in cryogenics deals with the tempering
(hardening) of metals. In the past, heat was applied as a tempering
agent, but it was found that tempering at low temperatures could work as well
if not better. Today everything from razor blades to brake rotors can be
hardened at low temperatures. In addition, cryogenics can be used to
"un-warp" a piece of metal prior to machining it. The removal of heat puts the
piece in its "most relaxed state".
Low temperatures are frequently used in the medical field from freezing
embryos to (killing) warts.
The space industry frequently uses liquid fuels (hydrogen) in their
rockets. They even pack liquefied oxygen to provide an efficient burn.
All these require very low temperatures.
One interesting field in physics deals with superconductivity.
Certain materials show no electrical resistance at low temperatures.
Medical MRI imagers require a super strong magnetic field to work. Some
of these devices create this field by passing currents through superconducting
wires (a huge electromagnet).
Cryogenic grinding. This is also known as cryomilling. This
process is cooling or chilling material and then reducing it into small
particles. Basically it is for material that is hard to mill down at normal
temperatures so it needs to be chilled by dry ice or liquid carbon dioxide to
be able to be processed into fine powder.
OK, want to take cryomilling one step further? Read this student
post: Cryogenics is being used outside the US as an alternative to cremating a
body. The process is called promession. The body is submerged into liquid
nitrogen which makes it so brittle it shatters into powder as the result of
slight vibrations. This is supposedly a "green" way of disposing of a body by
avoiding the release of pollutants into the air, and the remains degrade w/in
6-12 months after the procedure. It was invented and patented in 1999 by a
Swedish biologist, Susanne Wiigh-Masak. (The first facilities were scheduled
to be ready in 2009 in Sweden, Germany, Great Britain, South Korea, and South
Africa.)
www.en.wikipedia.org/wiki/promession
Hot Topics - Scientists have found ways of achieving very high
temperatures ... not hundreds but thousands of degrees and much higher.
Your mission is to post one important and/or practical application when things
get very hot.
This topic is open ended. You can only cool things down to absolute
zero (about -460 Fahrenheit) but how high is high (when discussing
temperature) is left to the imagination. I suppose you could say the
first and most important application of high temperature is fire.
Cooking comes to mind as an immediate application. However, let's push
the thermometer up a bit and see where it takes us.
- The process of applying heat to weld metals is over 3000 years old.
- Plasma and laser cutters can zip through metal like a scissors cutting paper.
- Surgeons use laser scalpels because the heat tends to reduce the amount
of bleeding since it naturally cauterizes severed blood vessels
- At really high temperatures, you can get simple hydrogen to fuse into
helium in a process known as nuclear fusion. This is what is happening
at the center of our sun to produce the energy we receive in the form of
infrared, visible and ultraviolet radiation. We have mimicked that
process in the hydrogen bomb but perhaps we will find a way to control the
process to provide our energy needs in the future.
-
Atom smashers at a U.S. national lab
have produced temperatures not seen since the Big Bang — 7.2 trillion
degrees, or 250,000 times hotter than the sun’s interior — in work
re-creating the universe’s first microseconds. Taken from
USA Today 2/16/2010
Pressure
- If the atmospheric pressure is so high, why doesn't it crush in your
skull?
Your head does not contain a vacuum (I hope) so the
pressure of the atmosphere is not acting against your skull unopposed. The
pressure inside your skull is at the same pressure as the outside. It all has to do with pressure differences!
You will notice any slight changes in pressure as an irritating or painful
discomfort in your ears. For example, if you take an elevator up a tall
building, you may notice the difference in your ears. A yawn may
equalize the pressure. You feel this when driving in mountains or in
airplanes. Who can forget Arnold Schwarzenegger's response to a sudden
drop in pressure in Total Recall? Getting back to reality, divers
experience great pressure increases over a very short distance. If a
diver goes just 34 feet down, he/she will experience twice the pressure
compared to the surface. The diver's lungs are compressed to half their
volume. Diving below 100 feet requires a slow ascent to the surface to
avoid "the bends" .. where nitrogen (which is diffused into your bloodstream
at that pressure) and needs to be released out of the blood slowly.
- A suction cup 6" in diameter is fastened to a wall. If the inside of the
cup holds a complete vacuum, how much force would you need to dislodge it?
The area covered is πr2
or 3.14 x 32 = 28.26 in2 assuming a surface
pressure of 14.7 pounds/ in2 we get 28.26 x 14.7 = 415 pound of
force
In 1654 Otto von Guericke (who invented the vacuum pump) performed an
experiment to demonstrate the power of a vacuum. Two steel hemispheres
(about 20 inches in diameter) were sealed by a vacuum. Even a team of
horses could not pull the two sections apart.
- Will a soda straw work at any length? Explain.
No! Look again at the way a mercury barometer
works. Imagine you were trying to drink some mercury from a straw (not a
good idea). If you were a perfect sucker (is one born every minute?),
you could only draw the mercury up about 30 inches because the atmosphere is
pushing it up the tube. Water is much less dense so a perfect vacuum
would allow the atmosphere to push water about 34 feet. You can bet that
miners who needed to pump out water knew this.
- From a physics point of view, what factor(s) determines the liquid
pressure at the bottom of a beer bong?
There is only one factor - height of the liquid column.
For example, if the column were 34 feet high, the pressure would be equivalent
to one atmosphere of pressure. OK, if you want to get technical, you
would have to take into consideration the density of the liquid but beer is
basically water. Also, you could argue the absolute pressure at
the bottom of the bong should take into account the pressure of the atmosphere
but I'm only concerned with the pressure difference due to the liquid.
My guess is that if you used one of these devices too many times it would
become difficult to answer any of these questions :)
- Will a depth gauge give different readings under fresh water vs. salt
water? Explain. If different, which would tend to give the higher number?
A depth gauge is really a water pressure gauge. Salt water is slightly denser than fresh water. As
a result, at any given depth the gauge would read slightly higher in salt
water. The difference in readings is not a significant factor for divers
... but sharks are. Note: Cargo boats have a "load line" (Plimsoll line) that indicates the
safe level for loading. If the boat is loaded in New Orleans at the
mouth of the Mississippi River, and you load exactly to this line, the boat
will rise up (above this line) once it sails away because the saltier (denser)
ocean water offers more buoyancy.
- You are under buoyant force right now! Explain. Give an estimate how
large this force is.
The buoyant force is equal to the weight of the
displaced fluid. You are displacing air right now so you would need to
estimate your volume and the weight of an equal volume of air.
Estimate: What is the volume of the human body? You can fill a huge
barrel brim full of water, climb in and submerge yourself under water.
Now measure the volume of displaced water (amount that flows out). I'll
try a slightly easier approach. I'll assume that the density of the
human body is slightly greater than water. Let's go 1.1 g/cm3.
A 150 pound person has a mass of 68 kg = 68,000 grams. Since D= m/V :
V = m/D (V = volume, m = mass, D = density) V = 68,000 g / 1.1
g/cm3 = 62,000 cm3 . Now the density
of air is 1.2 kg/m3 from
here.
Convert this to .0012
g/cm3 Now m = DV so .0012 x 62,000 = 74 grams
Wow ... all that work to find that you are "lighter" by about 3 ounces due to
the buoyancy of air. Don't worry, you will NOT have to do a calculation
like this on any test.
- A classic rock group called itself "Led Zeppelin". Could a zeppelin
constructed from lead actually fly? Explain.
Hey, steel is a whole lot denser than water yet steel
boats float on water. The key is to make sure your ship's total weight
is equal to the weight of the displaced liquid (water). Blimps only fly
if the same rule applies but now you are displacing air. A lead balloon
was actually made on the show Mythbusters: season 6 episode 2. All you need is a lead chamber holding a low density gas (hydrogen or
helium). I'm thinking how gold (which is almost twice the density of
lead) can be pounded into a very thin foil. Imagine helium filled gold
balloons by Donald Trump! I'm not a materials engineer but I know that lead is also very
malleable. Anyone want to make a lead zeppelin? If you do, maybe
you will find a "stairway to heaven".
One student commented that this makes the phrase "going over like a lead
balloon" an invalid statement!
- Water may not be compressible, but air certainly is. The density of air
is considerably greater at the surface than it is at higher elevations. How
does this affect the buoyancy of a zeppelin with a rigid hull? Is there a
limit to how high it can go?
Have you ever noticed that weather balloons are only
partially inflated when leaving the ground? This is because as the
balloon rises in the atmosphere, it encounters less outside pressure and
expands to create an equilibrium. With a rigid hull (like the Goodyear
blimp), the pressure within the chamber remains fairly constant (ignoring
temperature changes). It only has buoyancy as long as its total weight
equals the weight of the displaced surrounding air. However, the (fixed)
volume of surrounding air weighs less and less the higher you ascend into the
atmosphere. It eventually reaches a maximum height and can go no higher.
In the case of a hot air balloon (which you can think of as having a fixed
volume), you gain height by making the inside air hotter. This makes it
expand which forces air out of the balloon and makes the overall weight less.
The balloon rises until it moves into thinner air and a new equilibrium is
established. The air inside the balloon eventually cools and becomes
denser. This draws in surrounding air and adds weight to the system ...
down you go.
- How are submarines able to surface and dive (from a standpoint of
Archimedes' principle)?
Submarines have ballast tanks that are filled with air
or water. To make the sub surface, compressed air is fed into the tanks
and the overall weight and density of the U-boat drops. Remember, the sub will float
if the weight of the sub equals the weight of the displaced seawater. To
make the sub dive, the ballast tanks are flooded with water ... changing the
overall weight and density of the boat.
- Describe how the common pressure gauge known as a Bourdon gauge works.
You're likely to have a dial Bourdon pressure gauge
attached to any pressure tank. It works by the same principle as those
"party snakes" you blow on and a coiled paper tube extends out to annoy the
person sitting next to you. The gauge contains a thin hollow coiled tube
that tends to straighten itself when the pressure inside increases. The
change in the tube shape is transferred (via a linkage) to a dial reading.
The animation below should help you see how this works.
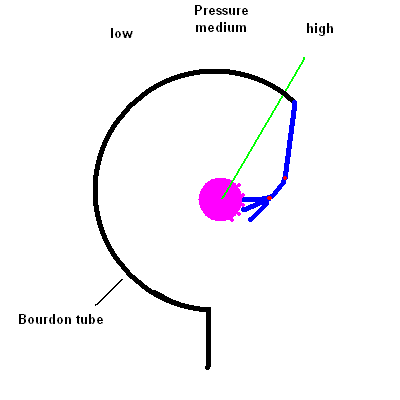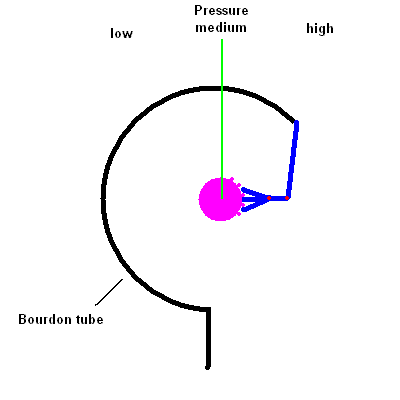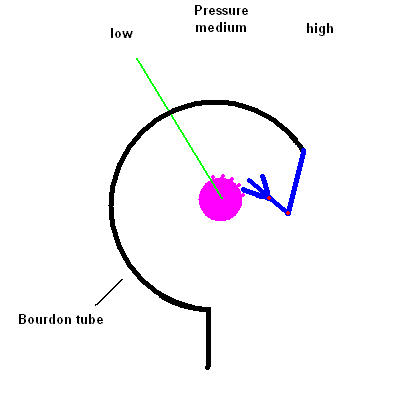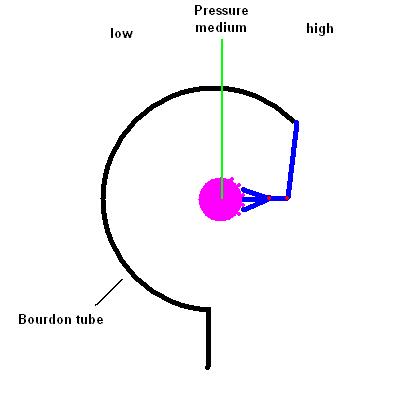
Bourdon Pressure Gauge animation
11. One process, called pascalization, is used in the food
industry. Explain what is involved here. Does it affect the taste of foods?
Does this process work on all foods?
Pascalization involves the use of
extremely high pressure to kill harmful bacteria in foods. The public
has seemed to reject the use of irradiated food (despite frequent stories of
contaminated food with deadly results). Pascalization offers an alternate
means for public safety. It does not alter the taste of food but only works
on certain foods like meat products but does not work in fruits and
vegetables (turning them to mush).
12. Why is it essential to “bleed” air out of your car’s brake line?
The hydraulic fluid in your brake
line is uncompressible .... meaning its volume does not change regardless of
the pressure. This is important because any pressure applied from
the foot peddle (and vacuum assist) can be transferred equally (via the
hydraulic fluid) to the brake pads. If there is a tiny air bubble
in the system, your brakes will not work because air IS compressible.
This means the volume will change (lower) when outside pressure is applied.
The necessary transfer of pressure from one system to another is greatly
reduced. Crash!!!
Applications of a Vacuum (many answers from students)
Just about
any spinning blade will create pressure differences. The household vacuum
cleaner creates positive pressure on the "bag side" and negative pressure on the
floor side. The same idea is seen in a shop vac, exhaust fan, blower fan
in your furnace, etc
Toilets in airplanes flush by a vacuum.
During flight, the air pressure outside the craft is considerably lower than the
passenger cabin. All you need is to tap that pressure difference to move
the waste materials to a holding tank ... at least we hope there is a
holding tank onboard :)
Respirators use both positive and
negative (vacuum) pressure to help patients breath. The
iron lung was one of the first applications.
Vacuum is used in the
technique called freeze drying. When the pressure drops below a
certain point, water boils (even at or below room temperature). This
drives the water out of food without cooking it.
When we heat water at
conditions where the pressure is lower than the atmospheric pressure, then the
boiling temperature will be lower. It works with other liquids too and can
be used to separate one component within a liquid from another. This is
because the different substances have different boiling points. This process is
called vacuum distillation. This process is necessary when liquid substances
need to be separated but they can be destroyed in high temperatures. The vacuum
distillation is most commonly used in the oil refinery industry.
There is an
entire industry devoted to making plastic things shiny! The process is called
Vacuum Metalizing and how it works is they literally melt metal until it
evaporates into a cloud. Then they use this "cloud" of metal in a vacuum
chamber along with the item to be coated and because of the vacuum, the
particles go straight to the object and go back to their solid state.
(Note from Jim: Good! However, the vacuum here is only used so the
cooler target is the only item the vaporized metal can condensate on. The
vacuum itself doesn't exert any force on the "cloud" to go one way or the other.
This process is very similar to the way dew forms on grass in the morning)
Vacuums are
used a lot in food packaging, for example, hot dogs.
A (partial) vacuum is
established inside a CRT (cathode ray tube). This is done to prevent
particle collisions. The idea is to direct a beam of electrons to the
phosphor coating on the inside of the television tube. Without a vacuum,
most of the electrons would never reach the screen.
A vacuum is used to remove
blood in a syringe. There is even a vacuum established in a test tube
known as a Vacutainer. Look for this the next time you donate
blood.
Small spider cracks
in a windshield can sometimes be repaired by applying a vacuum to extract air in
the cavity and allowing a resin to fill the void.
Vacuum leak testing is used
to detect minute leaks in welds, gaskets, seals, valves, etc. A certain gas
or liquid on the high pressure side diffuses through the (possible) leak and is
detected on the vacuum side with a sensor.
Auto body shops frequently
use a vacuum to pop out dents and/or create a suction cup on an area to apply
"pull points".
A thermos bottle uses a vacuum chamber to reduce the
transfer of heat via conduction (and convection) between the inside bulb and the
outside jacket.
In the manufacturing of a light bulb, a vacuum is use to
extract the combustible oxygen from the bulb which is replaced by low pressure
argon gas. Argon is used because it is non-reactive and it helps reduce
the "evaporation" of tungsten atoms off the filament.
The printing and
bindery industry uses necessary vacuum systems for lifting envelopes and pages.
Lumber is treated by submerging a
board in a bath of liquid preservative within a vacuum chamber. Once
the vacuum is released, the preservative is drawn deep into the wood.
NASA spent $23.4 million for a space
station toilet that uses a vacuum to draw 850 liters of air per minute (along
with some other items). For that matter, vacuum sewage systems are
routinely used on airplanes, trains and ships. There are several "large
scale" vacuum sewage systems in operation in Germany right now.
NASA houses the world's largest vacuum chamber to test Orion, which will take
astronauts on the next mission to space and the moon. This
chamber is designed to simulate the stresses of space on the space craft to
ensure that it will be able to withstand them.
The dentist or the dental hygienist will use a dental suction device on their
patient. This way they can suck the saliva, blood and liquid drugs in the mouth
of the patient.
In "clean rooms", there is often a carefully controlled air
flow which is controlled by a vacuum. In some places (like paint rooms)
there may be small prep room participants must first enter to remove loose
particles from their clothing. This room applies a slight vacuum to do the
job in the same way a vacuum cleaner works.
Vacuums are being used in the
medical field to help with healing different types of wounds. Applying a vacuum
on animal wounds have shown that blood flow improves and excess fluids are more
readily removed. This, in turn, helps in the removal of bacteria.
Source: The National Center of Biotechnology Information
http://www.ncbi.nlm.nih.gov/pubmed/15943495
You can use a vacuum to aid in
delivering a baby. Instead of using forceps, a suction cup is attached to
the head of the baby and out it pops.
http://www.aafp.org/afp/20000915/1316.html
After that baby is born, it
needs milk. A vacuum is used on cows as well as mothers to extract milk
for later consumption.
Finally, we have to mention the
Swedish-made
device Austin Powers had in episode #1 (but don't ask me if they really work
... they are not my bag, Baby).
 animation
animation