Key Terms
heat
kinetic energy
latent heat
Laws of Thermodynamics (First & Second)
potential energy
power
thermal energy
work
If you look up the word energy, you will likely see it defined as a noun - the ability to do work. I may not be grammatically correct, but I have always thought of energy as a verb. Energy implies action. The word is always employed when you observe something changing.
Matter can change many different ways, including:
Here are some forms of energy you should be familiar with.
Kinetic Energy - Energy of motion. The faster something moves, the greater its kinetic energy. The formula for determining an object's kinetic energy is KE = ½ * m * v2 where m is the object's mass and v is the object's speed.
The formula suggests that an object's KE is very sensitive to speed. For example, a car moving at 60 mph has 4 times the KE of a similar car moving at 30 mph (602/302) and twice the KE of a similar car moving at 43 mph. This is important if you realize that it takes gasoline to change a car's KE during acceleration. Maybe it is more important to know that cars must dissipate all their KE in collisions.
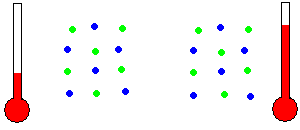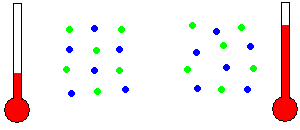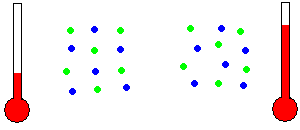
Thermal Energy - Thermal energy is often known as heat energy (although incorrectly in a technical sense). Two similar objects at different temperatures hold different amounts of thermal energy. The warmer object holds more thermal energy because the molecules within the object are moving in place with more KE than the cooler object. That is, the individual molecules are randomly wiggling around even though they are held in place by adjacent molecules. To be precise, heat is the transfer of some of this random molecular KE to/from the object as the object's temperature changes.

Temperature is related to molecular motion (animation)
Thermal energy can be tricky to understand because it can also involve a phase change (solid <---> liquid <---> vapor). Consider a solid which is just at its melting point. It takes energy to break the attractive forces which tie one molecule to another. If heat is added to this object, molecules use this added energy to free itself from its neighbor and the substance becomes a liquid. There still remains an attractive force with the collective (other molecules within the liquid), but now the molecule is able to move about. If heat is still added to the liquid, the individual molecules will pick up more KE (more speed) until it reaches its boiling point. Now any added heat goes to break the attractive forces again, and the molecules escape as a vapor. Now reverse the process, and you should see that heat has to leave the object if it moves from a vapor to a liquid or from a liquid to a solid. This transformation is called latent heat because it represents heat transfer without a temperature change.
Potential Energy - This refers to energy that is stockpiled for use at a later time. (This sounds more like a noun.) This can take many forms ...
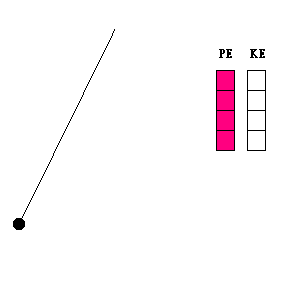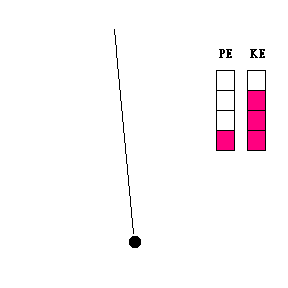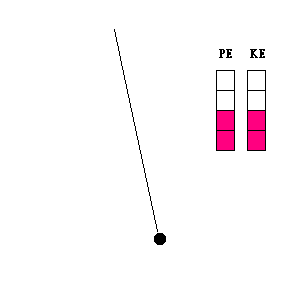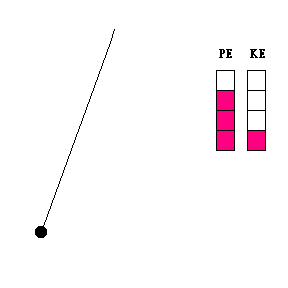
Transfer of energy (animation)
When a pendulum swings, it is constantly changing its height and speed. As it swings toward its lowest point, it is losing potential energy and gaining kinetic energy. The reverse is true when the pendulum bob moves upward toward the end of its swing. The constant exchange between potential energy and kinetic energy is experienced by any child who rides a skate board on a half pipe, goes on a roller coaster, or plays on a swing.
Question #1 - At what point in its swing would you expect the pendulum bob to be moving the fastest? The slowest? (Thought questions only ... not to be handed in. Click here for the answer.)
Do you own a "traditional" grandfather clock? If you do, you know that you have to crank up weights every few days to keep it going. As the clock runs, the weights slowly descend. The pendulum is merely a device which distributes the energy on a regular basis (keeps steady time) and works in conjunction with an escapement mechanism (link 1.3.a). In fact, some of the gravitational potential energy stored in the weights must be used to give the pendulum tiny pushes to keep it swinging, turn all the gears, and even play a chime every hour.
A very ugly "traditional" grandfather clock (animation).
Other devices that use gravitational energy would include a wrecking ball or pile driver. These devices deliver a huge force because they pump energy in a massive object ... slowly lifting it high above the ground. All this energy is converted to kinetic energy as the weight quickly descends to its target. Water towers are built high above the ground so they are able to store energy. At night a small pump works diligently to move water "upward" supplying every household with rushing water in the morning. Prior to the cannon, a device known as a trebuchet was the weapon of choice. It used the gravitational energy stored in huge counterweights to catapult stones at castle walls.

The water in this tower has energy by virtue of its height.

Cupid uses elastic potential energy (animation)
When you release an arrow from a bow, the arrow receives all its kinetic energy from the "elastic" energy stored in the bow. This elastic potential energy can be found in any device that is stressed. Other examples are seen in sling shots, balloons, and bungee cords. Probably the king of the hill here is the common spring. Springs can push (if compressed) or pull (if stretched). They have the ability to provide a small force if distorted a little, and larger forces if distorted a lot. Springs are used so often it would be impossible to do justice to them here. Just watch the many images with springs in following units. In most cases a spring is simply used to return a device back to a "starting position". You find springs in watches, music boxes, ball point pens, ... everywhere. Most garage doors use springs to counterweight the heavy door. Some use the conventional tension spring, and others use a torsion spring (where energy is stored by coiling or uncoiling the spring). Torsion springs are also found in the common mouse trap and even a clothes pin.
Here are some (torsion) springs found around my house.
When a gas is compressed, it stores elastic energy in the process. This energy source can be seen in paint ball guns (compressed CO2 cartridges), power tools driven by compressed air (pneumatic tools), and fire extinguishers. If your home gets water from a well, you likely have a "pressure tank" which is partially filled with air. When water is pumped to the house it goes to this tank and also compresses the trapped air in the process. This becomes the energy source to supply running water when the well pump isn't on. Ignited fuels create high pressure gasses which can push a piston in an engine or a bullet down the barrel of a gun. Liquids are very nearly incompressible so "elastic" energy can not be stored in them.
Similar observations lead to the concept of conservation of energy also known as the First Law of Thermodynamics. This concept states that energy can not be created or destroyed but only changes from one form to another. This is purely an empirical law ... meaning it is based on observation alone. However, since it was first conceived and observed countless times ... it has become a cornerstone of physics. In fact, numerous sub-atomic particles had to be "invented" to balance the energy equations and only later were they actually detected (neutrinos are one example). Astronomers have even "invented" a new form of energy (dark energy) to explain the strange motion of distant galaxies ... all based on the validity of this principle.
When you slide a book across a table, it comes to rest. Where did the KE of the book go? Recall that the book would naturally keep moving if not for the frictional force between the book and table top. This friction produces heat (both the book and table top become slightly warmer). In this case, the translational KE of the book is converted to random KE of individual molecules. The exchange of energy is from an "ordered" state to a "disordered" state. A scientist sees translational KE as "ordered" because all the matter is moving in one direction together. By the same token, heat energy is energy in "disorder" because the motion is random molecular movement. This leads to an observation dealing with the natural direction of energy transfer known as the Second Law of Thermodynamics. It states that in a closed system, the natural direction of energy transformation is toward a state of disorder. Example: translational KE to heat energy.
Did you ever observe a stationary book suddenly lose heat (cool down) and start moving across the table by itself? Not likely! But this would not violate the First Law of Thermodynamics ... but it would violate the Second Law of Thermodynamics!
Another example is seen in electricity. Electricity is the "ordered" movement of electrons through a wire. It should be obvious that this type of energy is extremely useful (we all use electricity), but left by itself, it would quickly get converted to useless heat as it encounters electrical resistance within the wire. (In all practical applications, you always lose some electrical energy to heat no matter what material you use as your conductor.)
Question #2 - Which direction does heat energy normally move – from hot objects to cold objects OR from cold objects to hot objects? Give several examples to support your answer. Click here for the answer.
Question #3 - What do you think the "natural" tendency is - the movement of a liquid from high pressure to lower pressure or from lower pressure to higher pressure? Give several examples to support your answer. Click here for the answer.
Question #4 - What is the natural motion of "free" electrons in a wire? Do they all move together in one direction or move in a random motion? Click here for the answer.
Why do we mention this? Because we are always trying to make things move ... cars, shafts, electricity in a wire, etc. That is, we place a high value on "ordered" energy because it can do useful things for us. However, if you look at how we typically accomplish this, we find it usually comes from heating something up (combustion in a cylinder or boiling water in a power plant). That is, most of your "ordered" energy originated from "disordered" thermal energy. Now think about what a refrigerator is trying to do. Doesn't it try to make something colder from something hotter? What do pumps do? Don't they try to make high pressure from something at a lower pressure? What do electrical generators do? Don't they make electricity (electrons all moving in one direction)? These are devices that appear to "buck" natural tendencies and in the process, become useable. This surely sounds like a violation of the second law of thermodynamics, but it really only APPEARS that way. You will just have to be patient for now and wait until this subject is reintroduced in a later unit to see why refrigerators and heat engines really do not violate this law.
Consider a heavy object at rest on the floor. You know that you have to add energy to lift this object to the top of a table. This would add to the object's potential energy. You could also push on the object horizontally and make it move along the floor. This would increase the object's kinetic energy (or add heat energy due to friction). In either case, energy must be added to the object from the outside in the form of work. When you apply a force on an object and displace it, you say you have done work. For work to be done, the motion of the object must be in the same direction as the applied force. Mathematically, work is the product of the applied force and the distance it moves (displacement).
Work = Force * Distance
Work is one way to easily see the quantitative aspect of energy transfer (although most students tend to dislike math). Don't worry; we won't make this too hard.
Example 1 - Lifting a box
Suppose you have to lift a 50 pound box from the floor to the top of a 4 foot high table. How much energy does that take? In this case, you know you have to exert a 50 pound force over a vertical distance of 4 feet. The result is 50 x 4 = 200 foot-pounds of energy. Not too bad so far.
You can get the same answer by considering the gravitation potential energy gain. Earlier we said PE = w * h and since the gain in PE comes directly from work done by the lifter, you should arrive at the same 200 foot-pounds.
Example 2 - Sliding the box
Now imagine you were to slide the box horizontally across the table top. Suppose you had to apply a steady force of 20 pounds to keep it sliding (against friction) and it was displaced by 3 feet as a result. The work done would be 20 x 3 = 60 foot-pounds ... still very easy.
Of course, there are many units for energy, and we have just introduced you to the foot-pound. Other commonly used units are BTU, and joules. See if you can use one of the many online conversion pages (see link 1.3.b) to convert 60 foot-pounds to joules. Answer: 81.349 074 joules
Example 3 - Dragging the box
Consider the situation where you tie a rope to the box and drag it across the floor as illustrated below:
You find it takes a force of 25 pounds in the rope (inclined at an angle of 30 degrees to the floor) to drag the box 2 feet along the floor. How much energy does this take?
OK, this one shows you the value of math. In order to solve this, you need to know how much of the 25 pounds acts in the horizontal direction. This requires trigonometry and is computed below.
A calculation shows
cos (30) = .866
25* cos (30) = 25 * .866 = 22 pounds (rounded)
This means that of the 25 pounds of tension in the rope, only 22 pounds is exerted in the direction that the box moves.
Work = Force * Distance
W = 22 pounds * 2 feet = 44 foot-pounds of work.
Can you see why skills in math may prove valuable? Don't worry, we tend to minimize the numbers in the class and stress the concepts. The concept here is that "work" is a form of energy transfer from one object to another. It shows that when a force is exerted on an object and the object is displaced as a result, energy must be provided. With moderate math skills, the amount of energy can be calculated.
Energy and power are closely related. Power is the rate at which energy is transferred. That is, time is the key to understanding the relationship between energy and power. Specifically:
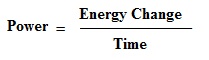
Power can be measured in familiar units such as watts or horsepower. Many textbooks use a different formula: Power = Work / Time which is certainly true but somewhat limiting since work is merely one form of energy transfer. Anytime energy is being converted, power can be calculated. For example, the sun's power output is 400 trillion trillion watts.
Consider a case where you lift a 100 pound barrel from the floor to the top of a table which is 3 feet above the ground. The barrel gained 300 foot-pounds of potential energy. Energy that came from you! If you complete this task in 2 seconds, your power output is 300/2 or 150 foot-pounds per second. A quick check at link 1.3.b shows that you just exerted about ¼ horsepower during the task (or about 200 watts of power). If you had a long plank to act as a ramp and rolled the barrel up to the table top, you still would have done the same amount of work (assuming no friction) but the task might have taken longer to complete. In this case, your power output would be lower. If done in 10 seconds, you would only require about 41 watts of power. Get the idea? If the plank is extremely long and the task is done over a much longer time period, the task requires less and less power (a smaller applied force over a greater distance exerted over a longer period of time). The next section introduces you to simple machines ... devices that can make us all seem like Superman.
Example 4 - In the Olympics, we watch world class athletes perform incredible feats of strength. Consider a weightlifter that can lift a 200 Kg mass to a height of 2 meters in ½ second. A simple calculation (and conversion) shows that this feat requires an output of about 10½ horsepower. Hint: use the conversion of 800 kilogram-force meter/second at see link 1.3.b. Don't expect this same athlete to put out this much power for very long. A healthy biker can exert about .3 hp for about an hour before exhaustion.
Example 5 - Now suppose this bike is hooked up to a generator.
Could an average biker be able to power a notebook computer (even though it
would be hard to type while biking)? Go to any online computer store and
look up the technical ratings of common laptop computers and convert this to
horsepower. I'll let you answer this one yourself. PS: Maybe
this would be a great way to get kids off TV :)
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author
Question #1: The pendulum will move fastest when the bob is at its lowest point. This is where the PE is the lowest.
Question #2: Heat energy moves from hot to cold until a state of equilibrium is reached (both objects are at the same temperature). One way is via conduction where heat moves along a stationary object. One example is seen when you place a spoon in a hot cup of coffee. Thermal energy moves from the spoon to the handle (along the spoon itself). Another way to transfer heat is via convection where thermal energy is transferred because a liquid or gas is set into motion. Air in your fireplace will move up the chimney because it warms, expands, and becomes buoyant. As it moves upward, it can cool as it exchanges heat with its surroundings, or cools via expansion. Another way to transfer heat is via radiation. The radiation from the sun (visible, infrared, ultraviolet, etc.) is one way for the sun to lose energy and for the earth to receive it. In each case, energy is moving from something that is hotter to something that is cooler.
Question #3: Let go of an untied balloon. The gasses will always flow out of the balloon because the tendency of any gas is to move from high pressure to low pressure.
Question #4: Left by themselves, free electrons will move in a random motion within a conductor. This is useless motion. However, if you can get all the free electrons within a wire to move together, you can do plenty ... we call this electricity.