As the temperature of a substance increases, the molecules that compose the substance move with greater kinetic energy. This forces the individual molecules to take up more space and the substance expands. If the substance is in a confined volume, the pressure can build to dangerous levels.
Key Terms
bi-metallic strips
conduction
convection
radiation
thermostat
Your car offers the perfect opportunity to learn some basic principles of heat transfer and thermal properties of matter. As your car heats up, the engine needs to be cooled. Heat is transferred, via conduction, from the engine block to antifreeze which runs through channels around the cylinder walls. Conduction is a process of heat transfer done on the molecular level. The image below shows that heat is conducted from hot to cold as molecules with excess kinetic energy share some of that energy with neighboring molecules. In domino fashion, thermal energy is transported, molecule by molecule, until equilibrium is reached.
Heat transfer via conduction.
A water pump circulates this hot antifreeze to the radiator. This transportation of thermal energy is known as convection. Convection can occur naturally (no pump needed ... as in warm air rising in a chimney flue) or can be forced (as we see here). Convection occurs when heat is transported via the motion of a solid, liquid, or gas.
Once the hot antifreeze reaches the radiator it is dispersed over a much larger area which greatly increases the rate of heat transfer via conduction to the air as well as through a process called radiation. As long as the radiator has a higher temperature than its surroundings, heat will be dispersed in the form of electromagnetic waves. In this case, the radiation is in the far infrared (invisible to the eye). A radiator painted black becomes a more efficient radiator than a shiny white radiator (it also becomes a better absorber of radiation ... meaning it would warm up better if cooler than the surroundings).
We will discuss these methods of heat transfer in other areas including ways of cooling the CPU of your computer or how an incandescent light bulb works.
Your car's cooling system includes a small device called a thermostat. The thermostat in your car keeps the engine working at a certain operating temperature (about 180-190 degrees Fahrenheit). When you first start your car and the engine is cold, a spring forces the thermostat to close and no antifreeze flows through the engine. Eventually things heat up and when the antifreeze reaches a certain temperature (say 185 degrees), the thermostat opens up and allows fluid to flow through the cooling system. The engine cools down. If the engine is running too cool, the thermostat closes up a bit, restricting the flow of antifreeze which causes the engine temperature to rise. But have you ever thought about how this small device works? A small container is filled with wax that melts at a set temperature. When it melts, it expands and exerts a large force (against the spring) on a small valve that opens the system.
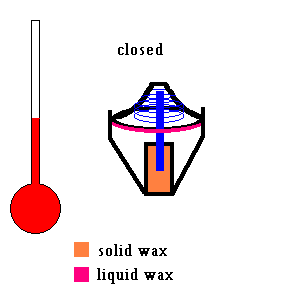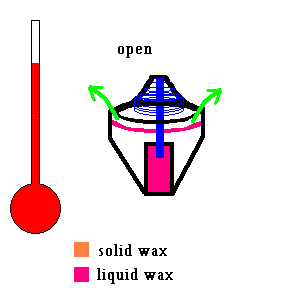
(animation)
Water is not like most substances because it expands (9% in volume) when it becomes a solid (ice). Most substances take up less space when they become solids. Why the difference here?
When a substance solidifies, the molecules arrange themselves in an ordered pattern known as the crystal lattice. Just as eggs come in cartons with the individual eggs closely packed, molecules usually form a compact arrangement. Hence, the substance contracts when it solidifies. However, water molecules form a 3 dimensional hexagonal ring when freezing (ever wonder why snowflakes usually have 6 branches?). This "hole" in the structure actually causes water to expand upon freezing. The expansion of water is both a good and bad thing. For one, it can cause enormous damage to roads and concrete when small amounts of water freeze in tiny cracks. The expansion is enough to widen cracks, form potholes, and tear apart enormous rock structures in a process geologists call "frost wedging". On the other hand, ice that forms on a lake will float and form a layer of insulation which protects marine life. Imagine what would happen if water contracted when freezing. Surface ice would become denser and sink. Rivers and lakes would eventually freeze from the bottom up, killing all plants and animals in the process.
Temperature can be measured many different ways, using:
Measuring temperature from the expansion of a substance is the most common and easiest to comprehend. The other techniques will be covered later as you gain more background knowledge.
The expansion of a heated liquid can be used to make a simple thermometer. As the liquid in the bulb reservoir warms up, it expands up a narrow calibrated capillary. Once mercury was used because it expanded linearly over a wide range of temperatures, but mercury is toxic and difficult to dispose of safely. Now a non-toxic liquid alcohol works just as well under normal conditions. This is the simplest way to measure temperature and the easiest to understand. The earliest thermometers were called thermoscopes. We can thank inventors such as Santorio, Galileo, and Fahrenheit for this device.
Different substances expand to different degrees when heated the same amount. Anyone who has installed vinyl siding is well aware that it will expand much more than its backing material on a hot day, so it must be hung loosely to allow for expansion and prevent buckling.
When you attach two different metals together and heat them up, one metal will expand more than the other and the strip will bend (see the animation below). This makes bi-metallic strips a useful tool. The simplest application is a thermometer (where the bi-metallic strip is in the shape of a coil). Many cheap oven thermometers are this type.
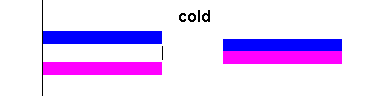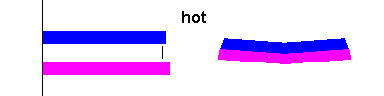 |
 |
Bi-metallic strips curve when heated (animation) Courtesy Wikimedia Commons
John Harrison was an English clock maker (horologist) in the 1700's who worked with pendulum clocks (grandfather clocks). He realized that the rate of the clock's movements was set by the length of the pendulum ... the longer the pendulum, the slower the clock would run. This presented a problem because as the temperature changed, so would the length of the pendulum ... and therefore, the clock's movement. Clocks in warm rooms would run slower than clocks in cold rooms. By using different metals, he made a compound pendulum which would not alter its length at any temperature.
Below are some ways the thermal properties of materials can be used.
Thermostats measure and control the temperature in your house. Thermostats can be mechanical or electronic. Older homes have mechanical thermostats which use bi-metallic strips (usually in the shape of a coil) to open or close a switch. Attached to the end of a bi-metallic coil is a glass bulb partially filled with liquid mercury and electrical contacts inside to activate the switch.
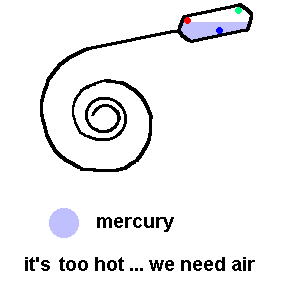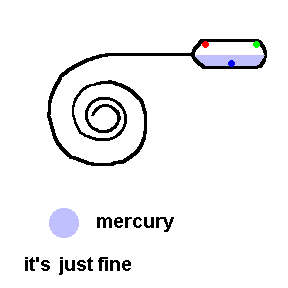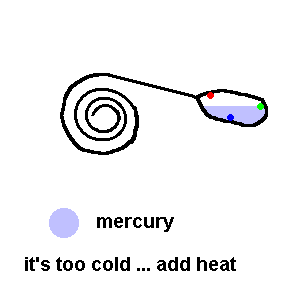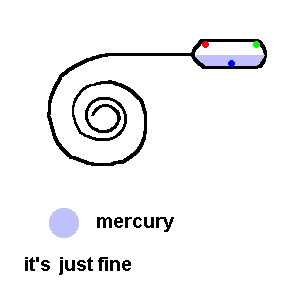
A common old home thermostat uses a mercury switch (animation)
Here the coil is really a bi-metallic strip which expands when heated. This animation shows that when it is too cold, the coil contracts and allows an electrical connection to the furnace (see contacts in the bulb). If it gets too hot, the coil tilts the tube so that the air conditioner turns on (contacts reversed).
Most electronic thermostats are programmable. They use a thermoresistor (thermistor) to determine room temperature and microcontrollers to switch the heating or cooling on and off. This is covered in the next unit.
Thermal circuit breakers use bi-metallic strips to trip a circuit when the strip carries too much current and overheats (because of electrical resistance) and breaks contact ... opening the circuit. This simple safety system is common on most hand held hair dryers.
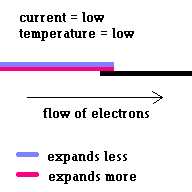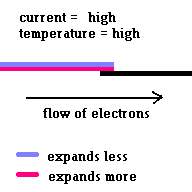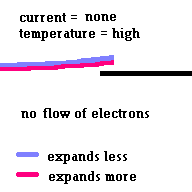
(animation)
The emergency sprinkler systems in most buildings also make use of thermal properties of matter. In some cases it is quite simple. When a fire starts, it simply melts a small bit of solder ... which releases high pressure water to the room.
In some cases, the high pressure water is restricted by a small glass bulb filled with a liquid. When a fire breaks out, the liquid in the bulb expands more than the glass confining it. This builds up tremendous pressure within the bulb and it breaks ... allowing the high pressure water to release into the room.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author