Key Terms
CMOS photo sensors
doping
inverter
LED
n-p junction
n type silicon
Photoelectric Effect
photon
p type silicon
valence electrons
wavelength
Isaac Newton (1642 - 1727) demonstrated that you can use a prism to break white light into its component parts (the visible spectrum). Conversely, he showed that the net result of combining all the colors of the visible spectrum (rainbow) is white light.
White light is the sum of all the rainbow colors.
Newton also debated (with Christian Huygens) about the nature of light, arguing that light was a particle. Huygens believed that light was a wave. No conclusion was reached at that time, but we now know that in some respects light exhibits wave like properties, and in other ways, behaves like a particle!
In 1801, Thomas Young showed, conclusively, that light behaved like a wave. In so doing, he allowed us a physical way to distinguish between the various colors of the spectrum - wavelength. Wavelength is designated by the Greek symbol lambda (λ) and is the distance from one wave crest to the next.
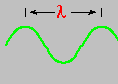
The wavelengths of visible light are extremely short, with red light slightly longer than that of blue.
![]()
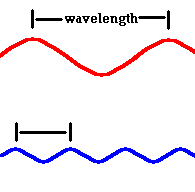
λ red light = .0007 mm λ blue light = .0004 mm
We now know that visible light is only a tiny section of a wide range of radiations which can not be seen with the eye. Thus, light is considered one (very small) part of the electromagnetic spectrum.
Edmond Becquerel, in 1839, first produced electricity from light although it took over 60 years before the process was understood. Becquerel demonstrated the Photoelectric Effect where electrons were ejected off a metal plate when subject to light of various wavelengths.
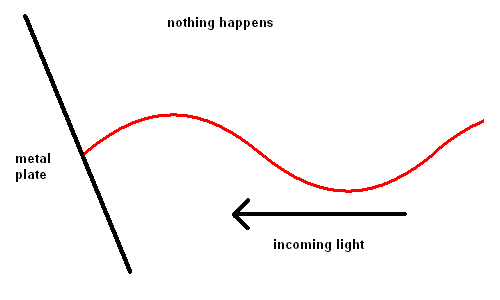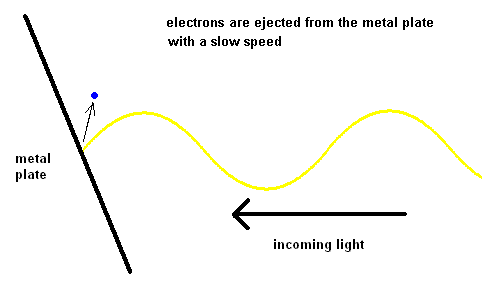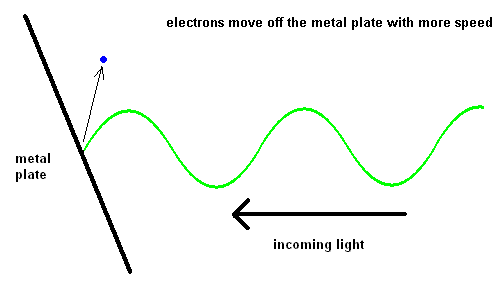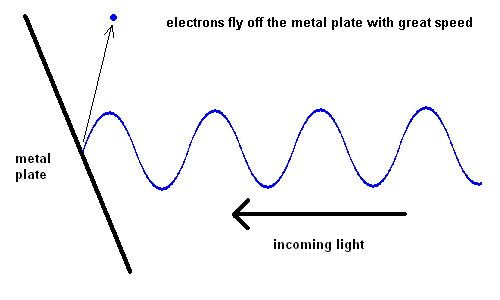
The Photoelectric Effect (animation)
For some strange reason, red light would produce no effect when striking the metal plate, no matter how intense the light. However, when yellow light was used, electrons could be seen moving off the plate slowly. The intensity of the light changed the number of electrons ejected, but not the speed. The shorter the wavelength of the light, the faster the electrons would move off the plate. This implied that blue light had more energy than red light.
The turn of the 20th century led to a new understanding in the behavior and structure of matter and energy. This revolution was led by Max Planck, Niels Bohr, Albert Einstein, and others. It became apparent that the energy in light seemed to act like tiny bullets which were eventually called photons. Einstein accomplished many great feats in his career, but most people don't realize that his only Nobel Prize (awarded in 1921) came from his explanation of the photoelectric effect (done in 1905).
We've come to realize that light is made up of "bundles" of energy (much like a particle) and the energy each photon contains is a function of its wavelength ... the "bluer" the color, the more energy the light contains.
The longer the wavelength, the lower the photon energy! Likewise, the energy in radiation with shorter wavelengths is high. It should be clear to you why ultraviolet light is something to avoid as well as excess exposure to X-rays and gamma rays. Astronomers have long known that gamma rays entering our solar system must have come from very energetic events such as supernovae.
The reason red light produced no effect on the metal was because the energy in these photons was not sufficient to dislodge an electron from its lattice structure.
The next quest is to find a way to harness this energy and convert it into electricity for practical use. For this we need silicon.
Most solar cells are made from the element silicon. Although some solar cells are now being manufactured out of other semi-conducting materials, let's start by looking at silicon technology first to understand the basics.
Silicon is the second most abundant element in the crust. It has atomic number 14 which means the nucleus contains 14 protons. A neutral silicon atom, therefore, has 14 electrons orbiting the nucleus. It is these electrons that play the major roles in chemical reactions and chemical bonds ... and not just any electrons, only the valence electrons need be considered here. What are valence electrons? As you put together atoms, the electrons fill in "shells". Once a shell is filled, the next electrons go to the next higher shell. In silicon, the two innermost shells are completely filled leaving the 3rd shell filled with only 4 "valence" electrons. To gain stability, atoms make every attempt to complete the octet (8 valence electrons) and do so by gaining or losing electrons (becoming ions in the process) or by sharing valence electrons with other atoms. This is chemistry 101.
In the case of silicon, stability can be achieved when one silicon atom shares its valence electrons with four neighboring silicon atoms forming a 3D crystalline lattice (see diagram above).
The first step is to purify silicon in a clean room. Since silicon in nature usually forms a natural bond with oxygen, this step is energy intensive and expensive as crystals are grown or cast. Once done, a wafer of pure silicon is ready for the next step.
Pure silicon is a poor conductor of electricity. Since all valence electrons in the lattice are "busy" holding itself together, there are no electrons able to move freely throughout the lattice. So after all the trouble of making the silicon 100% pure, the next step is to add some impurities ...but not much. One needs to add only 1 "outsider" atom for every million silicon atom in a process called doping.
In the classic example, one impurity is the element phosphorus. Phosphorus has 5 valence electrons, so when it is introduced to the lattice (in small quantities), the crystal structure looks pretty much the same except now there is one extra electron with no job to do! This doped silicon now becomes a fairly good conductor of electricity. Scientists call this an "n-type" semiconductor (for negative ... since electrons have a negative electric charge).
In another way, silicon can be made to conduct electricity. Instead of using phosphorus to add a free electron to the lattice, the silicon is introduced with trace amounts of boron. Boron has only 3 valence electrons, so when it is introduced to the silicon lattice, there is a "hole" which can be thought of as a place that any free electron can skip into. Since the hole represents a kind of "missing electron" it is called "p-type" silicon (for positive).
Things start getting interesting when "n-type" silicon is placed in contact with "p-type" silicon.
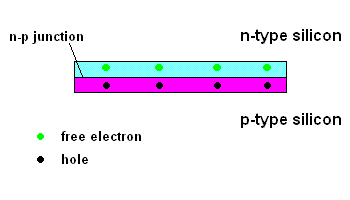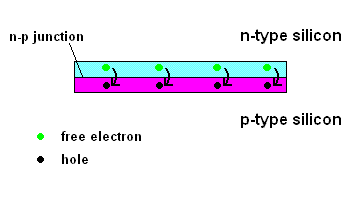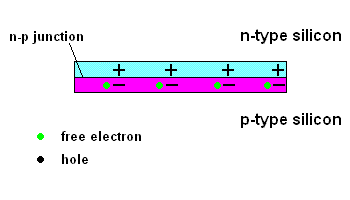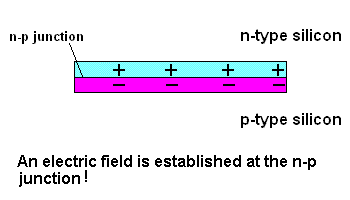
The n-p junction (animation)
Free electrons in the n-type silicon are free to move, and where is the perfect place for them to set up camp? It's in the "holes" found in the p-type silicon, of course. This means they cross the border (n-p junction), and when that happens, it leaves the n-type silicon with a net positive charge and the p-type silicon with a net negative charge. In other words, a permanent electric field is established at the n-p junction. This is important because any additional free electrons which find themselves near this junction will automatically be compelled to move in ONE DIRECTION ... away from negative (like charges repel) and toward the positive (unlike charges attract).
Enter the Photoelectric Effect! If the energy in light is sufficient to break the attractive forces that bind any electrons to their atom, the electron becomes free to move. If this occurs at the n-p junction, we now know which way they will move ... and when you get electrons all moving in one direction ....that is electricity (DC ... or direct current to be exact).
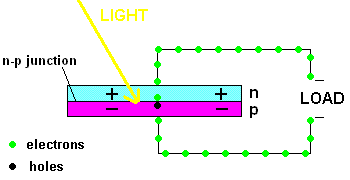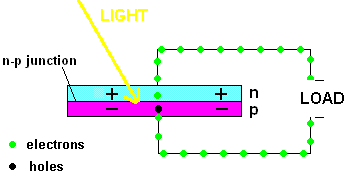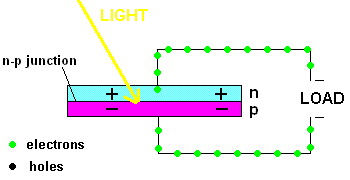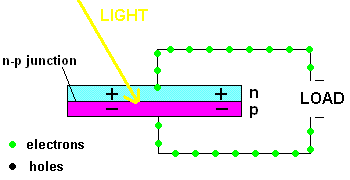
A PV cell in action (animation)
In fact, the light actually creates a free electron and a "hole" simultaneously, both of which are able to migrate under the influence of the electric field of the n-p junction. The electron heads toward the positive, and the hole migrates to the negative side of the junction. The animation above shows that the electrons move through the load and eventually meet up with a hole. The process continues as long as the light shines.
Note: The electrons don't actually "fly off" the p-layer as depicted in the photoelectric effect. They merely need to absorb enough energy to move from the "valence band" to the "conduction band". In physics lingo, this is known as the "band gap energy". However, for our purposes it is important to see that this energy comes from light.
A solar cell has a rather low efficiency of about 15%. That is, for every 100 units of energy that strike the cell, only 15 units result in useable electricity. There are some good reasons for that low number. Let's examine some of them below.
High Reflectivity - Silicon wafers are shiny! As a result, they naturally tend to reflect light. This represents wasted energy (since reflected light never enters the semiconductor) so antireflective coatings are used to offset this problem. This, however, adds to the overall cost of the cell and only reduces the effect, but does not eliminate it.

Photoelectric Effect (animation)
Bandgap Energy - Any energy below the bandgap energy (energy required to free an electron from the lattice) is unusable in a solar cell. Let us imagine that the bandgap energy of a solar cell lies in the yellow portion of the visible spectrum. In this case, only yellow light and any radiation with a shorter wavelength (higher photon energy) will be sufficient to free an electron from the lattice (free a valence electron to the conduction band). This means that any red light and all the infrared radiation is wasted because it hits the surface and does nothing. It gets even worse if we consider radiation with photon energies much greater than the bandgap energy. Say, for example, a photon of blue light hits the same solar cell. Yes, it has enough energy to free an electron from the lattice but any excess energy is converted to kinetic energy of the ejected electron ... which is quickly converted to waste heat ... again more lost energy. The overall problem is that any solar cell can only make efficient use of a tiny range of the electromagnetic spectrum. The rest is wasted.
A solution - One can construct solar cells that have different bandgap energies. Now layer the various cells (like stacking layers on a sandwich) so that each layer can take advantage of a small section of the spectrum. These are called multi-junction cells. Overall the efficiency will increase, but at great cost. This technique is used in special situations where the need for power overrides economics (application in space for example). Efficiencies of over 40% have been achieved using this technique.
Thermal Reduction - As panel temperatures rise, the overall efficiency of each solar cell drops. In Wisconsin, this may result in an annual loss of 14% in system efficiency.
Conduction Paths - Once a valence electron is free, it needs a path to the load. Thin wires on the top of the cell are required to carry current away ... which also tend to block more incoming light. The efficiency of a cell drops the farther a free electron must travel to a conducting wire, so lots of wires are needed ... but more wires block more incoming light. A catch 22 situation ... so a compromise is required which minimizes power losses.
In addition, there are power losses as a result of electrical resistance in the wires. This type of loss is common to all aspects of electricity, and it results from the fact that copper wires are not perfect conductors of electricity.
The sun doesn't always shine! - This is quite obvious. Even on a sunny day energy is lost by atmospheric absorption and scattering. The good news is that solar cells can take advantage of indirect light so they will work even on cloudy days (but not as well as with direct sunlight). Even reflected sunlight from the ground will increase the overall efficiency of a solar cell.
Solar panels need to be placed so they will receive direct sunlight for most of the day. This means your solar panels should not be blocked by shade trees. Also, let's not forget that it snows in Wisconsin, which means your solar panels will not work if blanketed with snow. Your system's yearly output will typically drop 2-5% because of snow cover.
Orientation - How you orient your solar cell to the sun will make a big difference in the output. It makes perfect sense that the best way to orient the cell is to have the sun's rays enter perpendicular to the surface. The overall intensity of light drops as the light enters at a glazing angle because the light is spread over a larger area.
Not only do solar rays change orientation throughout the day, they change throughout the season. A flat stationary horizontal panel will produce different outputs from a fixed panel tilted at the observer's latitude. The best results come from systems which track the sun so the rays always enter at the optimum angle, but this tends to get very expensive. Fortunately, the data has all been worked out and presented in the form of maps (link 4.1.a) which show how much solar energy will strike your solar panel for anywhere in the US.
In Wisconsin, a south facing location with a tilt between 25 - 45 degrees is recommended for any permanently fixed panels.
Inverter Losses - After you produce electric current from your solar cell, its waveform has to be modified for household usage. During this process, power losses occur (typically between 5-10%). The next section describes this process.
Time - Tests show that the overall efficiency of solar cells drops as materials degrade over time due to exposure to solar radiation.
Solar cells produce direct current (DC). That is, the electrons in the wires are moving in one direction. However, the current most devices in your home require is 60 Hz AC current. AC stands for "alternating current" which means the electron flow changes direction (with a frequency of 60 cycles per second). If photovoltaics are ever going to be part of our quest to get utilities away from burning fossil fuels to generate our electricity, we need to convert the DC current a solar cell produces to the more useful AC currents. This is a job for an inverter.
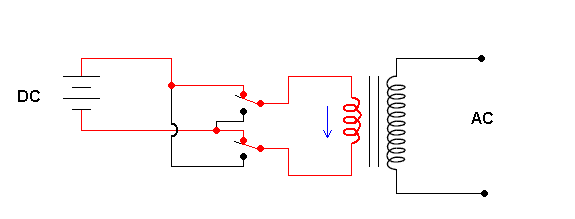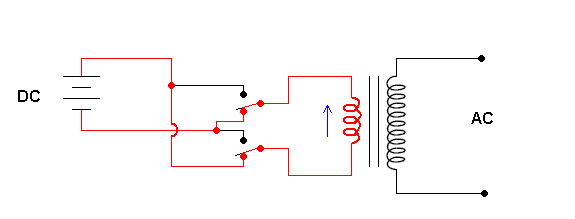
Schematic of an inverter (animation)
The animation shows that the key to an inverter is a switching mechanism. The switching is done by transistors (a great electronic on/off switch) but I've illustrated manual switches for simplicity. The contraption to the right is a transformer. This allows you to change the voltage and is covered in a previous section.
The output of these simple inverters is still not "user friendly". The output is AC, but in the form slightly different from the AC power provided by the utility. The inverter described above produces a "square wave", and the utility puts out a pure (true) sine waveform.
Perhaps you already own an inverter for your car or RV. These devices convert the 12 volt current from a car battery to 60 Hz AC. Just Google the words "power inverters" and you will find many manufacturers of these devices. The cheapest ones produce a "square wave", and care must be taken that the device you intend to power will accept that waveform. Slightly more expensive models advertise a "modified sine wave" output ... which is really a square wave with two "steps" or plateaus that better approximates a sine wave signal ... but this is still not a pure sine wave. Pure sine wave inverters use transistors as switches to chop and modify a single "square wave" into thousands of individual steps to mock the desired wave form. The exact procedure is omitted because it requires an advanced knowledge of electronics.
Your solar inverter should be a synchronous pure sine inverter. That is, a pure sine wave which is 100% in sync with the utility wave (both waves peak at the same time). This way, you can sell any excess power you generate back to the utility. WE-Energies currently sells power for about 13 cents a kilowatt-hour (2016). Click link4.1.b for current rates. Prior to 2016, WE-Energies was buying back power at 22½ cents per kilowatt-hour as an incentive to install these systems in homes and businesses but the rates have changed.. Click link 4.1.c for the current solar buyback rate.
My first practical application with photovoltaic cells came when I bought a solar powered calculator. The cost was low and the thing lasted forever (I still have it). This was a perfect application of photovoltaic (PV) energy because the device itself required very little power. It was very clear (back in the 70's) that the only places PV could be used on larger scales were in extreme applications. NASA turned to PV to power most of their satellites in orbit. The NASA rovers on Mars were solar powered as well. I remember seeing solar cars and even a solar airplane. This was pretty impressive, but not practical for you and me.
When I first saw some solar powered emergency call boxes along the highway in
the south-west, I realized that PV applications were about to turn the corner.
I became totally hooked on PV during a recent trip to Hawaii. I was
absolutely amazed at the number of houses with solar hot water and PV electric
systems resting on rooftops, but Wisconsin is not Hawaii!

Today you can purchase solar powered:
However, in Wisconsin I have not seen too many local large scale PV applications ... but only because I have not looked hard enough! Yes, PV is still quite expensive, but there is hope! The first baby steps will likely come from private industry and residential applications. As stated before, any excess electricity you produce can be sold back to the utility. Your electric meter actually spins backwards! WE-Energies and Focus on Energy have offered rebate incentives that have brought PV electric systems within reach of the average home owner and small business ... even in Wisconsin! The good news is Wisconsin IS making great progress generating electricity using wind turbines. The utility was required (under state law) to generate 10% of the state’s power with renewable sources by 2015 and they exceeded that goal!
Photovoltaic cells can do more than just capture light to make electricity. They can capture information. Consider a 2 dimensional array of photovoltaic cells arranged in rows and columns. If a single photon strikes any point of this grid, electricity will stream both horizontally and vertically from the target to a microcontroller. This gives the exact (X ,Y) coordinates of the photon strike. With sophisticated software, this grid not only can tell how many photons hit the grid, but what kind of photon as well (i.e., color). Probably the most common application is found in the digital camera. A 6 megapixel camera has 6 million separate photovoltaic cells which can capture, count, and classify nearly all the photons that hit the grid during the exposure.
What happens when you feed a document into your fax machine? An intense beam of light races across the paper! This beam is so fine that it takes many vertical scans just to "read" one line of text (depending on the DPI setting). This beam is reflected to an array of PV cells (arranged in a line). If your original fax is white, light will reflect to a cell and produce electricity. Where the paper is black ... no reflection ... no electricity. This pattern of light and dark is translated from electric pulses of "on" and "off". This electronic pattern is then converted to different tones for the phone lines. On the receiving side, the process is reversed ... tones are translated to electric pulses, which tells the printer to deposit ink or not.
Scanners work much like a fax machine, except to get a color image, the reflected beam is split into three parts ... and each separate beam goes to a different "filtered" sensor. The 3 filter colors are red, green and blue. That is, if the original has some red, only the PV cell with the red filter will record a "hit". We will see (in a later section) that you can combine these 3 colors in different ways to make almost any color you like.
One thing you should be aware of is the fact that many devices work in "reverse" to serve another purpose. The water wheel can be "flipped" to make a pump. An electric motor and electric generator are opposites. Turn the principles of a battery around and you can electroplate something. In a photovoltaic cell light hits a surface and frees electrons (and holes). What happens if you force an electron and a "hole" together (across a diode)? You guessed it - light!
Devices that do just that are called an LED for "Light Emitting Diode". Basically a battery provides the force necessary to compel electrons to combine with "holes" at the n-p junction. This liberates energy in the form of light. Early calculators all used LED's before liquid crystal displays became popular. Most likely you are sitting in front of a computer with LED power indicators shining (as well as an LED screen) ... cell phones light up with LED's ... almost all electronic gadgets contain LED's. These units are cheap to build, last a long time, and require little electrical energy to use. You can currently see LED's at work in traffic lights, tail lights and anywhere you need a small "on" indicator. As stated before, you can combine red, green, and blue to make just about any color you wish. Thomas Edison's light bulb will go the way of the dinosaurs and slide ruler. I don't think we will have to wait too long for this. I have LED light bulbs in my house right now ... what are you waiting for?
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author