
Key Terms
blackbody
continuous spectrum
electromagnetic spectrum
filament
frequency
halogen lights
incandescence
Kelvin temperature scale
Stefan-Boltzmann Law
visible light
wavelength
Wien's law
In the 17th century two great scientists, (Newton and Huygens) argued this point (to no definitive conclusion). Newton argued that light was a particle and Huygens contended that it was a wave (much like the waves you find in water). In 1801, Thomas Young showed, conclusively, that light behaved like a wave. A little over a century later, quantum theory showed that light has properties of a particle. As it turns out, modern physics has shown that light does, indeed, have properties of both waves and particles.

Courtesy Wikimedia Commons Courtesy Wikimedia Commons
When Isaac Newton passed white light through a prism, it separated into a "rainbow" known as the visible spectrum. Since one color blended into another without any gaps, this has also been called a continuous spectrum.
A continuous spectrum from white light
Light is a wave ... called electromagnetic radiation. It consists of an oscillating electric and magnetic field that travels through empty space. This radiation moves incredibly fast in a vacuum ... 186,282 miles each second. The speed of light is designated by the letter c and all the colors in the visible spectrum travel (in a vacuum) this same speed. What, then, distinguishes one color from another? One answer is the wavelength, designated by the Greek letter lambda - λ. This is the distance between successive wave crests. Red light has a slightly longer wavelength than blue light.
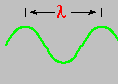
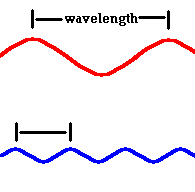
Red light (at one end of the visible spectrum) has a longer wavelength than blue light. However, another way of distinguishing between the different colors of light is by their frequency, that is, the number of waves that pass by a point every second. The animation below is designed to show that red light has a lower frequency than blue light. Imagine you were able to count the waves as they pass by the vertical line. Since both red and blue light travel at the same speed, you would find more blue waves passing the vertical line each second than red waves because the blue waves are closer together.
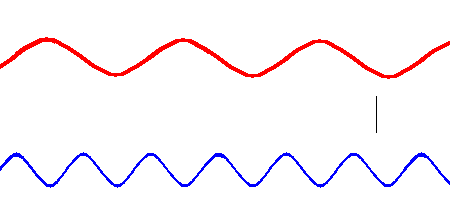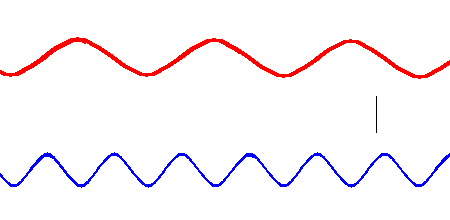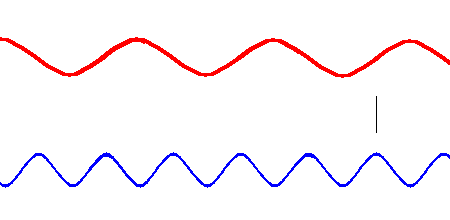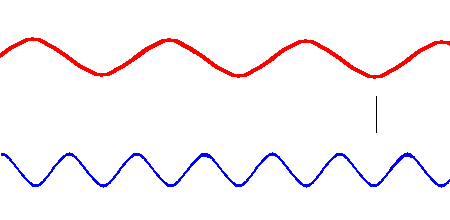
Red light has a lower frequency than blue light. (animation)
The relationship between frequency (f) and wavelength (λ) may be expressed in the following equation:
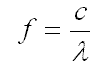
Where "c" is the speed of light. Notice that as wavelength increases, frequency decreases.
But there are waves with higher frequencies (shorter wavelengths) than blue light and waves with lower frequencies (longer wavelengths) than red light. These radiations are invisible to the eye, but exist in nature. Together they form the electromagnetic spectrum. See the image at link 5.4.a
| Wave type | Wavelength | Frequency |
| radio | very long - several meters | very low 106 Hz |
| microwaves | ||
| infrared | ||
| visible | Red light = .0007 mm or 7000 Å | |
| Blue light = .0004 mm or 4000 Å | ||
| ultraviolet | ||
| x rays | ||
| gamma rays | very short (a few Å ... or the size of atoms) | very high 1019 Hz |
Note: Å stands for angstrom units. An angstrom is a very small distance. 1 meter = 10,000,000,000 Å also Hz is a measure of frequency. It is short for Hertz and translates to "waves per second"
Just beyond the visible spectrum there exists electromagnetic waves which have slightly longer wavelengths than red light, and are known as infrared radiation. Although we can't see this radiation, we can feel it in the form of heat. On a warm summer day you can feel the warmth of the sun on your skin. This is coming from solar infrared radiation. Your own body radiates quite a bit of infrared radiation, which can be detected by "heat scopes". This is often depicted on TV and in the movies where someone can spy on people in the dark with infrared goggles. One of my favorite movies, Predator link 5.4.b, tries to convey the fact that the alien is adapted to view an infrared universe (not a visible one).
 Here
is how the "Predator" would see me ... an infrared image of me.
Here
is how the "Predator" would see me ... an infrared image of me.
With a wavelength just slightly shorter than violet, ultraviolet radiation is also invisible to our eyes. Everyone should be aware that the sun emits UV, and that wearing UV blocker will protect you from this harmful radiation. Sunburns are caused by UV damage to your skin cells. In fact, you should avoid any types of radiation with short wavelengths ... especially prolonged exposure to X Rays and certainly Gamma rays (just ask The Hulk)
Here are some main points you need to understand:

This light bulb has holds the world record for duration (since 1901)
Courtesy Wikimedia Commons
![]()
A continuous spectrum (the spectrum of white light)
A filament brain teaser: Consider two standard incandescent light bulbs (image below) which are identical in every way except the filament in bulb B is much thicker than the filament found in bulb A. Which bulb do you think will be brighter and why? Make a guess before reading on.

This question is equivalent to asking: What is the difference between a 50 W bulb and a 100 W bulb? We know that a 100 W bulb appears brighter, ... and it is consuming more electrical energy (in the same period of time) ... remember the Watt is a unit of power. The key lies in the filament itself!
The electrical power consumed is the product of the voltage and the amperage, ...
P = VI or Power = Voltage * Amperage
In most cases, there is very little we can do to alter the voltage (V) since it is set by the electric company at 115 Volts (unless a dimmer switch is involved). So to produce a higher power rating, we need to increase the current (amperage) through the filament. That is, we need to make "I" bigger. Ohm's Law tells us that:
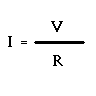
All we can do here is change the resistance (R) of the filament (again because V = 115 volts). To increase the amperage, we need to reduce the resistance! This can be done in one of two possible ways ... shorten the length of the filament ... or increase the thickness of the filament (because a thicker filament offers an easier electrical path for electrons). Going back to the two bulbs pictured above, we are given that the two bulbs are identical in all ways except the thickness of the filament. Therefore, bulb B will be brighter than bulb A since it has the thicker filament. Did you guess this correctly?
Below is a blackbody radiation curve similar to the one produced by the sun. Note: The outer sun (photosphere) is not a solid but a hot compressed gas ... which also produces a continuous spectrum.
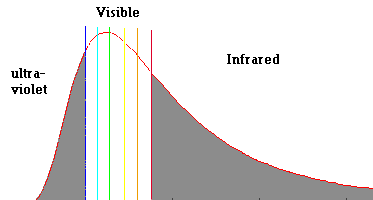
Radiation Curve of the Sun's Photosphere (a hot compressed gas)
The image plots intensity (Y) against wavelength (X)
Notice that the sun produces roughly the same intensities of all the spectral visible colors. This is why the sun looks "white" to the eye ... (do not look directly at the sun). Also note that the sun produces quite a bit of radiation in the "invisible" ultraviolet and infrared. Our next step is to try to understand the nature of this radiation curve and to see how this curve changes if the temperature changes.
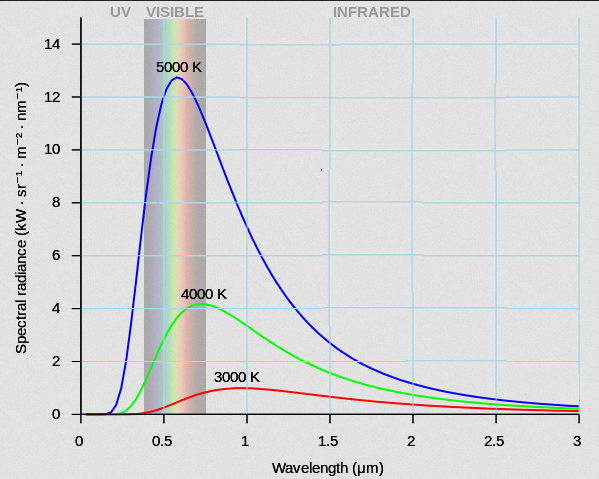
Courtesy Wikimedia Commons
This chart compares the radiation curves of three similar hot solids of different temperatures. There are 2 important differences that need to be pointed out.
Observation #1: The hotter the object becomes, the more energy it radiates (per unit area) each second. That is, the intensity gets bigger ... at every wavelength. This has been summarized in a law known as the Stefan-Boltzmann Law. It says that the energy output of a radiator increases as the temperature increases according to this equation.
(T is in Kelvin -see below)
Simply stated, the hotter, the brighter!
Observation #2:
As the
temperature increases, not only does the radiation curve grow in size, but the "peak" radiation shifts toward shorter wavelengths.
That is, cooler objects put out more radiation in the infrared. Hotter
objects put out peak radiation in the visible. Really hot objects
produce their maximum intensity in the ultraviolet.
Simply stated, a filament that is 5000 K will put out a higher percentage of light in the visible than a 3000 K filament. It is not just a coincidence that our eyes are able to detect electromagnetic radiation in the same region that our sun has its peak output. We view the visible because the sun radiates the most in the visible!!!
This has been summarized in a law known as Wien's Law.
where T is temperature measured in Kelvins -see below, λ (max) is the wavelength where the radiation curve peaks and is measured in angstroms)
In the case of the sun whose photosphere (surface) temperature is 5750 K, we get a peak amount of radiation at a wavelength of 5026 Angstroms (plug 5750 for T in the equation above). This wavelength lies right in the middle of the visible spectrum.
Wien's law gives astronomers a great way to determine the temperature of stars and other celestial objects. By rearranging this equation, you find:
All you need to do is scan the spectrum of a star and determine where it emits the peak radiation (λmax) and apply the formula. For example, an astronomer can analyze a blue star and determine that it puts out its peak radiation ( λ max) at a wavelength of 2890 Angstroms. From this, they would know that the photosphere (surface) of that same star is 10,000 K. Blue stars are hotter than our sun. Likewise, red stars are cooler than our sun.
You may have been confused by seeing all the temperatures listed in K. What is a K??? To honor Lord Kelvin, a temperature scale was created where the zero point is absolute zero. Absolute zero is the coldest anything can get and it is very cold ... -460 Fahrenheit or -273 Celsius. Deep space (space far from any stars) is very cold ... only 3 Kelvin. You thought Wisconsin was cold?
All this talk about blackbody radiation and the nature of incandescence actually started a revolution in physics. If you have noticed, I never actually talked about why hot objects (or hot compressed gasses) emit electromagnetic radiation, let alone why they produce the aforementioned blackbody curves. This is exactly what physicists were trying to determine at the turn of the last century. Classical theory at that time predicted radiation curved wildly different from experimental results. They assumed that the vibrations (or oscillations) of atoms resulted in the emission of radiation. That is partially true but theory did not match reality. This became known as the ultraviolet catastrophe (see link 5.4.c). The solution came from Max Plank in 1900 when he made some crazy assumptions about the nature and behavior of matter at the atomic level ... namely, he assumed that atoms emit energy in discrete amounts known as quanta. What??? Ok, without turning your brain into jelly ... this means the atoms can't just wiggle at any frequency but are only allowed to vibrate with discrete values. If he made that assumption, he was able to derive an equation (link 5.4.d) that would precisely match data taken in the lab. Little did he realize but he gave birth to a field of physics known as quantum mechanics. The story of the light bulb and Max Plank is explained in greater detail in link 5.4.e. It actually took others like Albert Einstein and Niels Bohr to put all the pieces together. This is where the particle properties of light come into play. That part of that story is introduced in the next section on fluorescent lights.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author