Your food is here:
Key Terms
compressor
condenser
expansion valve
evaporator
latent heat
refrigerant
Keeping food cold not only preserves it longer, but often makes it taste better ... as any beer connoisseur can attest. Before modern refrigeration, ice cutters would saw blocks of ice from frozen lakes and rivers in winter and insulate them for storage. The "ice man" would deliver blocks to your home. My wife still refers to her frig as the "ice box". Life got much easier with refrigerators.
A refrigerator is simply a heat engine in reverse. In all heat engines, work is done by tapping a temperature difference. That is, by allowing heat to flow from a high temperature source to a lower temperature reservoir, you can divert some of that energy to do useful work. A refrigerator is just the opposite. By putting in some work, you can transport heat from a lower temperature reservoir to a high temperature reservoir. Refrigerators are "heat movers"! That is, they remove heat from food inside the unit and transport it into the room at a higher temperature. How do you remove heat from something that is already cold? By making it even colder! The trick is finding a way to make this happen, and the trick is making a substance (a refrigerant) go through a phase change.
Your refrigerator cycles a substance called a refrigerant. The refrigerant is the key to the unit because it is a substance with a very low boiling point. Earlier refrigeration units used sulfur dioxide and Freon as refrigerants, but modern industrial units use ammonia (poisonous to humans) and home units use hydrochlorofluorocarbons and hydrofluorocarbons (which are environmentally safer).
When any substance goes through a phase change, energy is involved. When a liquid evaporates, energy is required to break molecular bonds that exist between adjacent molecules. The liquid can obtain this energy by absorbing heat from its surroundings. For example, if you allow rubbing alcohol to evaporate from your skin, you feel a cooling sensation as heat is removed from your body. This is called latent heat of vaporization.
Let's start by looking at the cycle a refrigerant goes through in your refrigerator.
Your food is here:

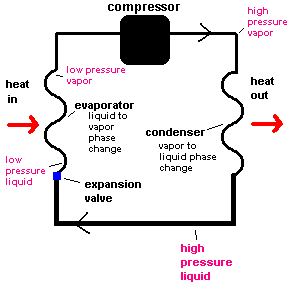
Since this is a cycle, we can start anywhere and eventually come back to the same place. So let's choose to start at the very bottom (of the diagram) where the refrigerant is a high pressure liquid which is moving through coils inside your refrigerator. The high pressure refrigerant encounters a small opening similar to a tiny puncture hole in a scuba tank. The refrigerant squeezes through this hole (called the expansion valve or throttle valve) and finds itself in a low pressure environment. This area is called the evaporator because as the liquid moves through the region, it changes to a vapor (evaporates).
In physics, a picture known as a "phase diagram" can be used to show what happens when the pressure (P) suddenly drops as it moves through the expansion valve. The red arrow shows that the refrigerant can no longer exist as a liquid and seeks to become a vapor. It is the same thing you would see if you were to suddenly expose the high pressure butane in a lighter to atmospheric pressure. The butane would quickly boil away.
This change of phase (liquid-vapor) requires energy the same way you have to add energy to water as it boils to a vapor ... only the refrigerant boils at a much lower temperature. So where does the "boiling" refrigerant get the needed energy? From your food! As thermal energy is transferred to the evaporator coils, your cold food gets even colder.
By the time the refrigerant is ready to leave the evaporator, it is all vapor ... and still at a low pressure. The compressor (which is that big black unit near the bottom of your refrigerator) does just that ... compresses this vapor to a much higher pressure. This is like reversing the red arrow in the phase diagram. The vapor seeks to become a liquid, but this demands that some energy be released as the refrigerant condenses to a liquid. This is accomplished as the refrigerant moves through a coil appropriately known as the condenser. These coils are located on the back or bottom of your refrigerator (and collect a lot of dust which should be removed occasionally). You can easily feel the heat released from these coils as the refrigerator is running. As the refrigerant makes its trip through these coils, all the vapor will be converted to a high pressure liquid. We are back to the place we started ... and the cycle continues.
The key to understanding the workings of a refrigerator are:
An air conditioner works on the same principle. The only difference is the evaporator coils are found on the inside of your home and the condenser coils are exposed to the outside.
If interested, click link 5.2.a and read this article about refrigerators or watch the animation at link 5.2.b
If interested, click link 5.2.c and read about air conditioners.
Your home may become too humid in summer (or you have a damp musty basement). A dehumidifier is designed to remove water vapor from the air. Actually this unit is nothing more than a freezer with the door open. Water vapor in the air will condensate on the evaporator coils (much like water vapor condenses on your windows in winter). This water runs to a pan or a drain.
Heat pumps are nothing more than an air conditioner on a grander scale. In summer, the coils exposed to the inside of the house become the evaporator (taking in heat) and the coils exposed to the outside become the condenser (giving off heat). However, a heat pump can also act as a heating device (in winter) by simply reversing the process. In winter, the "outside" coils become the evaporator (taking in heat) and the "inside" coils become the condenser (giving off heat).
Heat pumps don't work very well in Wisconsin because overall efficiency drops as the temperature difference increases (inside temperature vs. outside temperature). However, in areas where climates are more moderate (say Kentucky), they become practical heating/cooling devices.
If you have a camper, you may have a small refrigerator that runs off LP gas. This is an ammonia absorption refrigerator which is a bit different from the one in your house. They have no moving parts (other than the moving fluids) and require no pump to move the fluids because they move by buoyancy and gravity. However, they still use the concepts of latent heat to produce cooling. Below is a simple diagram showing the way fluids move in the system.
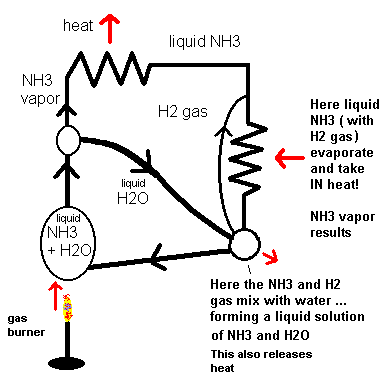
Basically it works by using heat from an LP burner to vaporize liquid ammonia (NH3) in water (H2O). When the ammonia condenses, it releases heat to the outside. The liquid ammonia then flows by gravity and mixes with hydrogen gas (H2) inside the cooler. This environment compels the ammonia to vaporize where it absorbs latent heat from your food. The diagram (above) shows how ammonia, water and hydrogen gas cycle through the system.
Thermoelectric Coolers
In an earlier unit we covered the science of thermocouples (Unit 3 - Electrochemistry). If you recall, the key to a thermocouple involved the electric potential when dissimilar metals meet at a junction. If two such junctions were at the same temperature, no voltage was established. However, if one junction was at a higher temperature than the other, a net voltage was established across the system. It was found that a thermocouple can run in reverse! That is, if a voltage is applied across a series of junctions, one of the junctions would heat up and the other would cool down! This is known as the Peltier effect. This idea is applied in thermoelectric coolers which can cool or warm food off a 12 volt power source. They are commercially available now.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author