
Key Terms
exponential decay
fission
fusion
half life
isotopes
nuclear transmutation
radioactive decay
radioactivity

Ernest Rutherford Courtesy Wikimedia Commons
From this experiment, Rutherford drew these conclusions:
He was right. It took another 10 years for Rutherford to discover the proton and it wasn't until 1932 that Chadwick discovered the neutron. After that, the nature of the atom was becoming clear. The nucleus contains protons and neutrons. The mass of a proton is 1837 times as massive as an electron and the mass of a neutron is slightly greater than that of a proton. In terms of relative size, an analogy would work well. If you placed the nucleus of the atom at second base of Miller Park, the electrons would orbit in the parking lot.
Have you ever wondered why the nucleus is able to hold itself together? After all, neutrons have no electric charge so there appears to be nothing acting as glue holding them together. Even worse, protons have a positive electric charge and you already know that similar electric charges repel. If left to electric charges alone, the nucleus should fly apart ... yet it doesn't. There is a missing piece to this puzzle - a nuclear force. Actually scientists have discovered two types of nuclear forces - the weak and strong nuclear force. We need not distinguish between the two. The nuclear force is very strong ... much stronger than the electric force but only acts for very short distances. That is, if two protons were very close, nuclear force greatly overcomes electric repulsion. However, should these same two protons find themselves separated by some distance, there are no nuclear forces acting on them and they will repel by electric repulsion. This means there is a tremendous amount of energy associated with the nucleus in the form of "binding energy" due to these nuclear forces.
The number of protons in the nucleus determines which element we call it. Any atom with one proton in the nucleus is hydrogen ... 2 protons makes helium ... 26 protons we call iron ...92 protons becomes uranium, etc. This is true regardless of the number of neutrons found in the nucleus. Let's consider the hydrogen atom. Most hydrogen found in nature has a nucleus with only one proton. However, it is entirely possible for a hydrogen atom to have one proton and one neutron (which we call deuterium). Hydrogen can even have one proton and two neutrons (now called tritium). But these are all considered isotopes of hydrogen.
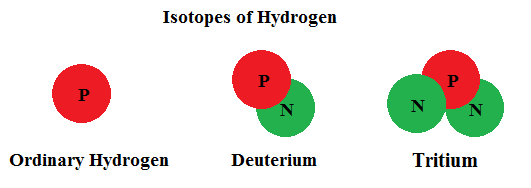
Isotopes of an element may be extremely stable or unstable. Unstable isotopes are radioactive. That means, over time, they spontaneously convert themselves to a more stable form. In so doing, they will emit particles or radiation (alpha particles, beta particles, or gamma radiation). An alpha particle is the same as the nucleus of stable helium. A beta particle is just an electron. You already know what gamma radiation is. When an isotope is radioactive, the net result is a change in the makeup of the nucleus. The nucleus can merely convert to another (more stable) isotope of the same element OR change from one element to another (called nuclear transmutation) by altering the number of protons. Either way, the nucleus of a radioactive atom emits energy as it tries to achieve stability.
The story actually gets bit more interesting. Albert Einstein realized the energy released in the process of nuclear transmutation was associated with a loss in overall mass. His famous equation. E = Mc2 is probably the most famous equation ever written.
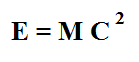
What Einstein was saying when he wrote it was that mass is merely another form of energy (something unheard of in his day). The equation itself just converts amounts. During nuclear transmutation, a small amount of mass disappears from the universe and is converted into pure energy.
When you make popcorn, you can never tell which individual kernel will pop first or when any specific kernel will pop. However, you can say a lot about the pattern of popping overall. You know that the action starts out slow and gradually builds to a maximum pop rate .. and then it quickly tapers off again. Think of this pattern as "popcorn decay"... as individual kernels change. The same things happens with a sample of radioactive material. The decay rate of a group of radioactive atoms is well established, and is known as exponential decay.
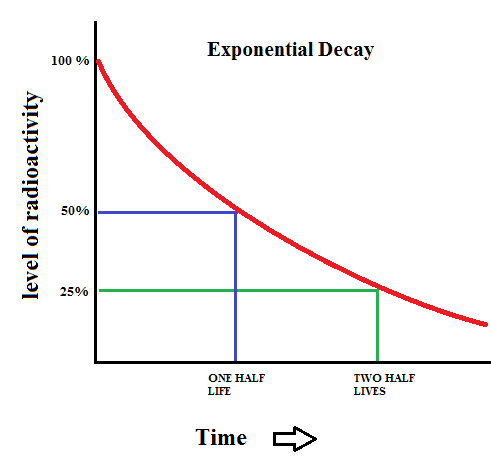
One of the aspects of an exponential decay is a quantity known as the half life. The graph above shows that after a time period of one half life, the level of radioactivity is reduced to 50%. After two half lives, the sample is only 25% radioactive. This pattern continues which yields a curve that never reaches zero.
Radioactive decay releases energy. The main reason the interior of the earth is so hot is because the Earth formed from several radioactive elements. There is so much heat (that tries to escape to the surface) that it is enough to drive plate tectonics. This, in turn, creates volcanoes, earthquakes, and mountain ranges. If nature uses this energy, why can't we? We have. The first attempt to use this energy was in the destructive power of the atomic bomb. Physicists during the second world war found ways to "stabilize" many radioactive atoms of uranium all at once. That is, they found that you can greatly accelerate radioactive decay by exposing a critical mass of uranium with a beam of slow moving neutrons. When one uranium atom absorbed a neutron, it would become so unstable that it would split the nucleus into two more stable nuclei ... emitting a lot of energy plus 3 more slow moving neutrons. This produced a chain reaction that quickly consumed all the uranium in the system. The result of the Manhattan Project was the atomic bomb. Shortly after, nuclear fission reactors were built that were able to release this energy at a rate faster than nature but much slower then a bomb. Fission is a process of splitting a heavier atom into lighter ones. Link 6.6.a does a nice job explaining this.
There are about 100 nuclear power plants in operation in the US (2016). Some of the electricity running your computer likely came from the Point Beach nuclear power planet (link 6.6.b). They work by speeding up the radioactive decay of uranium 235 (which is a rare unstable isotope of uranium). The heat produced is used to generate steam which spins a turbine connected to a generator. Generating electricity from nuclear energy is highly controversial ... especially since the disasters at Chernobyl, Russia in 1986 (link 6.6.c) and Fukushima, Japan in 2011. One of the byproducts of nuclear fission is highly toxic and radioactive plutonium (with a half life of 24,000 years). Another factor is the tremendous cost to build a nuclear power station. After three decades, there are only two new nuclear power plants being built in the US and scheduled to go online near the end of this decade. Link 6.6.d
Doc Brown came back from the future with Mr. Fusion.
What a great movie series!
Link 6.6.e

Courtesy Wikimedia Commons
So what is fusion? It is just the opposite of fission. Nuclear fusion involved joining atomic nuclei together into a heavier element ... releasing energy in the process. This is more than just theory, it has already been done ... in the form of a hydrogen bomb. Before that, our sun has been fusing hydrogen into helium for about 5 billion years. The energy released is the reason we have sunshine. Every second, 600 million tons of hydrogen is being converted into helium in its core (with a mass loss of 4 million tons every second). Those numbers sound big to us but they are chicken feed for the extremely massive sun. Fusion is good! Will we ever have Mr. Fusion??? I hope so, but we are very far from that day.
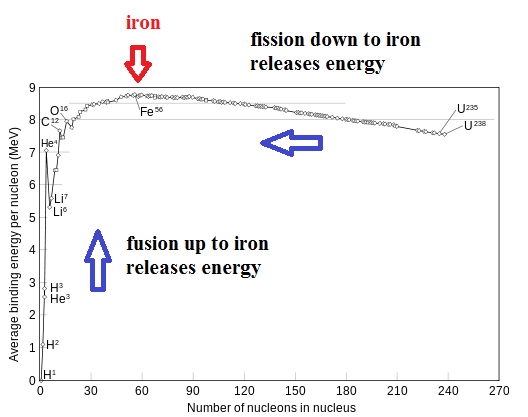
Maybe a bit of explanation is required here. How can you split up an atom and release energy (fission) and also join atoms together and release energy (fusion). This sounds like a trick. The solution is found when you look at the nucleus of an iron atom. The nucleus of an iron atom is the most stable of all the elements on the periodic table. Iron has atomic number 26 ... meaning the nucleus has 26 protons. Physicists say the nucleus of iron has the highest "binding energy" of all the elements. This is a bit like saying a ball on the ground has the lowest gravitational potential energy. If you want to lift the ball to a higher elevation, you need to put energy into the system. Likewise, if you want to transform the iron nucleus into a different element, you also need to add energy into the system. Only, you have two choices here. You can transform iron into something lighter or into something heavier .. but either way, you need to put in energy. Now let's look at the reverse situation. As long as you take a heavier element and move it towards iron, you will release energy. You can also take a lighter element and move it towards iron and release energy. In other words, you can release energy as long as you join lighter elements or split heavier elements.
Courtesy Wikimedia Commons
The use of radioactive substances can be used in the medical field for research, diagnosis, and treatments. There are tools (such as X Rays, or proton beams) where an external source of energy is used for medical applications. Nuclear medicine relies on a radioactive source inside the body for diagnosis and treatment. The primary diagnostic advantage of this technique is that the procedure provides information on the function of a body part being targeted. Is your heart pumping properly? Is your thyroid overactive? Do you have liver cancer? Your body behaves differently when a disease is present. The trick is to inject a radioactive tracer whose motion within the body can be monitored to look for signs of abnormal behavior which may be attributed to a disease or defect.
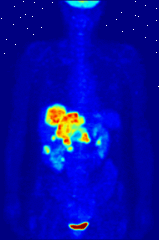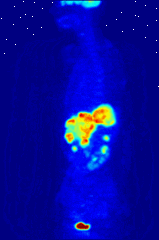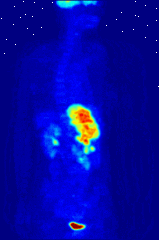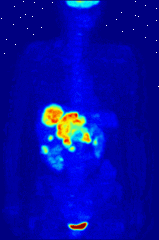
A PET scan Courtesy Wikimedia Commons
As far as treatments, patients with cancer tumors may be treated by surgically implanting a radioactive "seed" directly in the tumor and killing it. For example, prostate tumors have been treated with radioactive iodine-125, palladium-103, or iridium-192 for many years. However, a whole field known as targeted radionuclide therapy aims to destroy cancerous cells by finding clever ways to deliver radioactive materials directly to the tumor. One approach is to deliver these radioactive bombs by riding piggy back on specific antibodies that seek out cancer cells. Once at the target area, high doses of short range radioactive emissions (alpha or beta particles) kill only the cancer cells. This "seek and destroy" approach on the cellular level has shown great promise in the treatments of several cancers.
Naturally occurring radioactive materials break down into other materials at known rates. Radioactive parent elements decay to stable daughter elements. Once this rate is known, geologists can estimate the length of time over which decay has been occurring by measuring the amount of radioactive parent element and the amount of stable daughter elements.
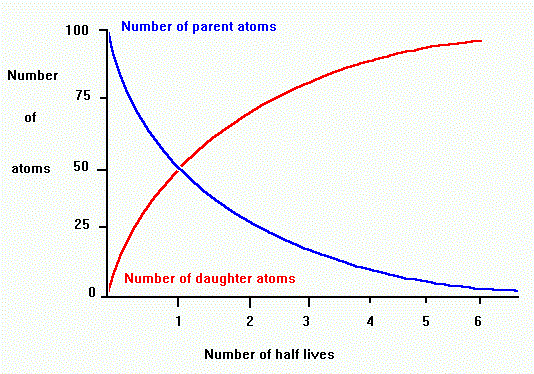
Each radioactive isotope has its own unique half-life. A half-life is the
time it takes for half of the parent radioactive element to decay to a daughter
product. The proportion of parent to daughter tells us the number of half-lives
the process occurred,
which we can use to find the age in years. For example, if there are equal
amounts of parent and daughter, then one half-life has passed. After two
half lives, the ratio of daughter/parent is 3 to 1.
Radioactive Parent |
Stable Daughter |
Half life |
|---|---|---|
Potassium 40 |
Argon 40 |
1.25 billion yrs |
Rubidium 87 |
Strontium 87 |
48.8 billion yrs |
Thorium 232 |
Lead 208 |
14 billion years |
Uranium 235 |
Lead 207 |
704 million years |
Uranium 238 |
Lead 206 |
4.47 billion years |
Carbon 14 |
Nitrogen 14 |
5730 years |
There are now over forty different radiometric dating techniques, each based on a different radioactive isotope. Each one is appropriate for a given time interval depending on the half life.
The Carbon 14 test is commonly used to date organic material such as wood and bone. C14 is produced naturally by cosmic rays entering our atmosphere and interacting with atmospheric nitrogen 14. All living organisms take in carbon (mostly carbon 12) but some of that carbon is a small amount of this radioactive isotope (C14). When the organism dies, it can no longer take in additional Carbon 14, so the amount decays over time, producing nitrogen 14 as a daughter product. After 5730 years, half of the C14 is gone, after two half lives (11,460 yrs), 75% of the C14 is gone, etc.
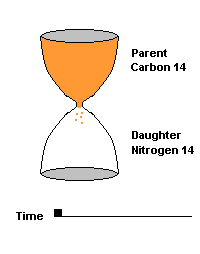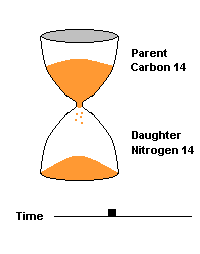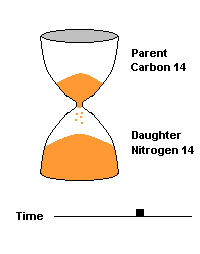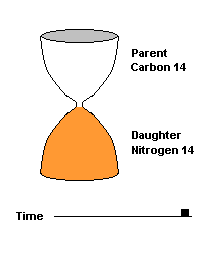 (animation)
(animation)
This hourglass animation is a good analogy to help you understand the principles involved. Suppose you walk into a room that has an hourglass already going. You can estimate how long ago the hourglass was turned by observing the amounts of sand left in the top (parent isotope) and how much has already moved to the bottom (daughter isotope). Of course, you will have to know the basic properties of that particular hourglass (which can be done at a different time) ... does it really take an hour to empty? But once done, you have a pretty good idea how to use this device to estimate the passage of time. The hourglass analogy breaks down at the very end because, in theory, there will always be some sand in the top half (parent isotope) of the hourglass.
The carbon 14 test does have limitations. For example, the Carbon 14 test would only be accurate for more recent times ... within 70,000 years (since it has a rather short half life). That is, after about 70,000 years there would be so little C14 left in the sample that it would be difficult to accurately measure. So you can see that you could not, for example, use the Carbon 14 isotope to date something like a dinosaur bone (over 65 million years old) but it would be very appropriate for dating a piece of wood found in the pyramids. Another problem arises because scientists have to estimate the initial amount of carbon 14 trapped in the body when it dies. The sun does vary in the amount of cosmic rays it produces over time, so the amount of C14 in the atmosphere also varies slightly. These variations are well known over the past 6000 years (because C14 is actually trapped in tree rings) but before that, it has to be estimated.
This technique is only valid for igneous rocks and the event being dated is when the rock was formed (crystallized) from magma or lava. If a rock re-melts, the radiometric clock is reset. Fortunately, there are enough radioactive isotopes with a wide range of half lives so that just about any igneous rock can be accurately dated. And there are several different tests that can be performed on one sample, so that if all the tests provide the same date of origin, you can be reasonably sure that the age is correct.
In 2014, scientists claimed that they found evidence of a rock dating 4.4 billion years old. This is, by far, the oldest dated rock (the oldest before this find was about 3.8 billion years). This crystal could only have formed at much lower temperatures than thought possible at this time in earth's history. What was interesting about this find was that there was evidence that liquid water was present at the time of formation. This was quite a surprise and offers clues that the earth had oceans and continents .... and even could have supported life at this very early time! Click link 6.6.f to read the story.
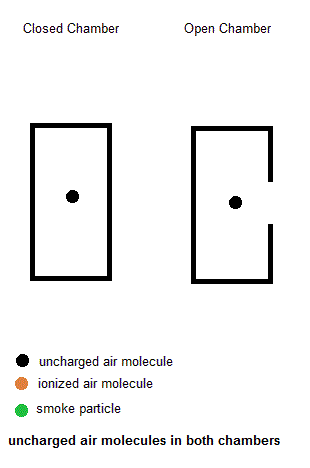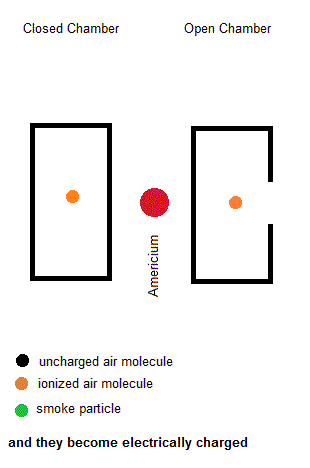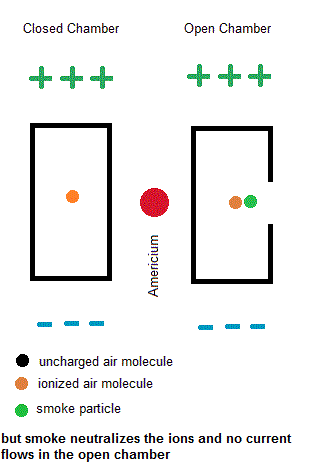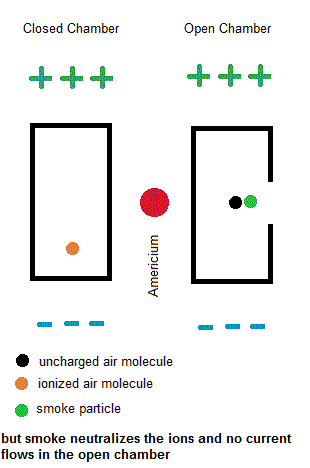
Ionization smoke detectors make use a radioactive substance (usually Americium-241 with a half life of 433 years). This radioactive substance emits alpha particles which hold a positive charge. Inside your smoke detector are two air chambers ... one open to outside air and the other sealed from outside air. Both chambers are constantly exposed to a stream of charged alpha particles from the Americium-241. As such, the alpha particles are able to ionize some of the air within both chambers which give some of the air molecules a net electric charge. A small voltage is also established across each chamber so the charged air molecules move ... constituting an electric current. Under normal conditions, the currents in both chambers are equal. However, if a fire is present, the charged smoke particles neutralize most of the ionized air, but this only happens in the "open" air chamber. The currents in both chambers will no longer be equal so a microcontroller will signal a piezoelectric buzzer to wake you up.
©2001, 2004, 2007, 2009, 2016 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author