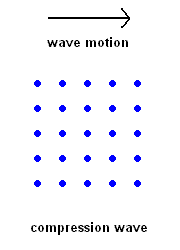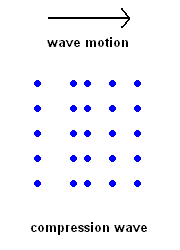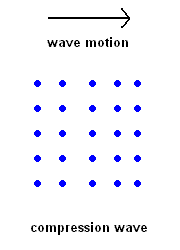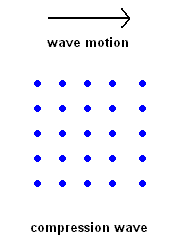
Compression Waves (P wave animation)
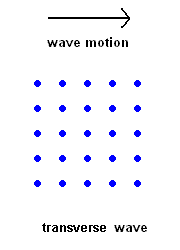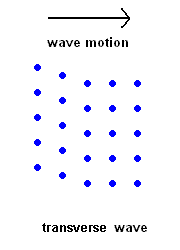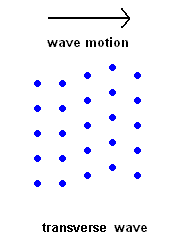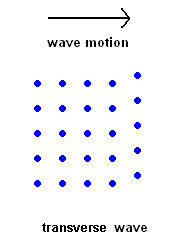
Transverse Waves (S wave animation)
Key Terms
absorption (dark line) spectra
Doppler blue shift
Doppler Effect
Doppler red shift
echolocation
electron microscope
MRI (Magnetic Resonance Imaging)
radar and sonar
X-ray crystallography
Introduction
You already know that sound and light are waves. We use our ears to detect sound waves and our eyes to detect electromagnetic waves (visible light). This you already knew. So let's talk about some things you probably didn't know. We can tell where a sound is coming from because there is a very slight delay between the arrival time of the sound wave to each ear. Our brain translates that to a direction. The retinas in the back of our eyes are made up of two different kinds of light detectors - rods and cones. When we look directly at an object, the inverted image projects to the center of the retina. That area of the eye is populated with cone receptors. They are sensitive to high levels of light and allow perception of colors. When you look at an object peripherally (off to the side), the image projects near the outer edge of the retina. This area is populated with rod photoreceptors. These cells are sensitive to low levels of light and give no color vision (yes, everyone is colorblind to some extent). The rods are also sensitive to motion ... probably so you can more easily detect if someone is sneaking up on you from behind. We use our eyes and ears as probes to learn about our environment. So let's "probe" a bit deeper to see the many other ways we learn about our surroundings using waves.
When a wave hits something, one of three things can happen:
The way the wave interacts with the objects can give clues about the nature of the object itself. Below are some ways that waves can be used as probes.
The crust of the earth is made of brittle plates which move with respect to each other. As they move past each other, energy is built up in the rock in the form of elastic potential energy. When the rock faults (breaks), all the elastic energy stored in the rock is released (like cutting a tightly wound spring) - this makes an earthquake! We discussed much of this in a previous unit.
The study of earthquakes is called seismology, and a person who studies them is a seismologist. As the earth shakes in an earthquake, a seismograph records the various waves set up.
Geologists have determined that there are distinct vibrations that occur during an earthquake. These waves penetrate deep in the earth's interior and enable seismologists a way to map regions we cannot possibly investigate first hand.
|
Compression Waves (P wave animation) |
Transverse Waves (S wave animation) |
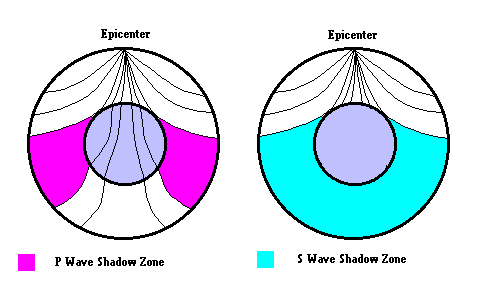
Discovery of the Earth's Core (Gutenberg Discontinuity) - A seismic discontinuity was discovered by Beno Gutenberg based on "P wave shadow zones". Gutenberg (1914) realized that there were "zones" where P Waves did not appear. Based on this, he surmised a major boundary existed which separated the mantle and the core. Later, the study of S Waves also showed shadow zones indicating that the outer core must be liquid (since S waves cannot travel through liquids).
Discovery of the Inner Core (Lemann discontinuity) - In 1936, Inge Lehmann used seismic records of an earthquake and noticed faint arrivals of p-waves in the P-wave shadow zone where no arrivals were expected (due to refraction at the core-mantle boundary). She realized that these signals represented P-waves that had reflected off an inner part of the core that was denser than the outer liquid core and suggested, therefore, that the core was composed of a solid nucleus and a fluid outer layer. The quest continues. In 2008, geologist Xiaodong Song confirmed the speculation of the existence of an "inner" inner core ... click link 6.4.a Also, it is now possible to make 3D maps of the interior of the earth.
Seismologists aren't the only ones who get valuable information from reflected waves.
|
Venus as seen in visible light (Credit NASA) |
Radar image of Venus (Credit NASA) |
Astronomers routinely bounce radio waves and laser beams off planets and the
moon to determine their exact position.
Ultrasound waves are high frequency waves of
compression which reflect off a fetus. Hospitals use ultrasound
to determine the sex of unborn babies and to see if potential problems may
exist prior to birth.
Fish detectors and more sophisticated devices can be used to map the
topography of the ocean floor using sonar.
Geologists set off surface explosions to reveal geologic
structures in the rock strata which could hold petroleum.
Cars have backup sensors to detect if you are about to hit
something. A beam of ultrasonic sound waves are sent out and if there is
an obstacle in the way, a reflected beam is detected ... alerting you to
observe caution. Self driving cars are constantly sending and receiving
reflected waves to avoid obstacles.
Have you gotten a speeding ticket lately? One technique to catch
people with a "lead foot" is to send out a laser beam, let it bounce off your
bumper and return to the sender. The round trip time is measured and a
distance is quickly calculated. A short time later, the process is
repeated ... which yields another measurement of distance. To turn this
into a speed, you find the change in distance and divide it by the time
between readings. If you read on, the next section shows how the Doppler
Effect can also catch you speeding.
LIDAR
(which
stands for Light
Detection and Ranging) is a relatively new technology where pulses of
laser beams are used as an echolocation probe to map the
surface of the earth. When used in conjunction with GPS data, this
system can generate highly detailed 3D contour maps. For example,
drones have been used to produce LIDAR images of ancient Mayan structures
and settlements previously unknown. LIDAR maps of the ocean floor and
coastal waters have been useful for navigation and also resulted in several
new discoveries.
Police use radar to catch speeders by sending out a signal (with a certain frequency). They then measure the frequency of the reflected waves to see how fast the object is moving (in your line of sight). This is known as the Doppler Effect (link 6.4.c ... which lets you move the rocket or asteroid to see the effect). This effect can be demonstrated using sound (a car moving past you produces a distinct drop in pitch) or electromagnetic waves (used by astronomers, meteorologists, and cops ... to see how fast things are moving).
 (animation)
(animation)
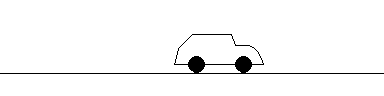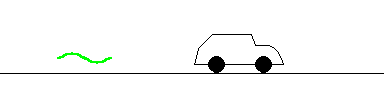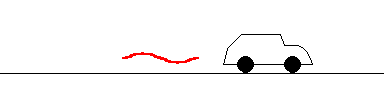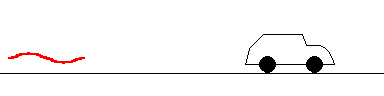
If a wave with set frequency (wavelength) reflects off a stationary car, it returns with the exact same frequency (wavelength). The police can detect this and see that the car is not moving at all. However, ...
 (animation)
(animation)
If the car is approaching the police, the reflected beam is distorted with a slightly higher frequency (shorter wavelength) than the original beam. The amount of distortion tells the police how fast you are approaching. This is called a Doppler "blue shift".
 (animation)
(animation)
The same police can bounce a beam off a receding car, but this time the reflected beam returns with a slightly lower frequency (longer wavelength). This can also tell the police how fast you are moving and is known as a Doppler "red shift".
Note: In these examples I've used visible light when, in fact, the police use a wave in the radio range of the electromagnetic spectrum. If visible light were used, you could only detect a change in color if the object was traveling near the speed of light. The instrument police use is sensitive enough to detect the smallest change in frequency. The faster the car is moving, the greater the change in frequency. Some Doppler radar uses lasers to set the trap.
Edwin Hubble used the Doppler Effect (on distant galaxies) to show us that the universe is in a state of expansion. He noticed that the farther a galaxy was from us, the greater was the Doppler "red shift".
Weather forecasters use Doppler radar to show us if there is any "large scale" circulation in the atmosphere. This can give meteorologists a 20 minute warning prior to the formation of a tornado.
Doctors can use the Doppler Effect to see if blood is speeding up or slowing down as it moves through veins and arteries. This gives them clues as to where obstruction might be.
Sometimes a wave is transmitted through a transparent object, but the object interacts with the wave by absorbing some of the incoming radiation. This can give us clues about the nature of the object, the chemical and physical properties of the object, or simply alert us that the object exists in the first place.
White light produces a continuous spectrum, with no gaps in the spectrum as you observe from red to violet. Low pressure gasses produce a bright line spectrum (as discussed in vapor lamps). However, there exists a third type of spectra, known as an absorption or dark line spectra. In order to make this kind of spectra, two things have to happen at the same time.
There needs to be some object capable of producing a continuous spectrum in the background.
A low pressure gas has to lie somewhere between you and the object making the continuous spectra (in the line of sight).
Dark line spectra of hydrogen
In this example, hydrogen selectively absorbs photons of the light passing through it, and uses the energy to excite electrons to higher orbits. Please review the Bohr model of hydrogen which was discussed in vapor lamps. Since hydrogen only emits 3 different photons in the visible spectrum when it releases energy, it stands to reason why hydrogen will only absorb the same photons (in the visible) to move electrons to higher orbits. As a result, the presence of hydrogen reveals itself in the form of this spectrum.
The solar spectra looks like this
In 1802, Wollaton discovered dark lines in the solar spectrum, and in 1817, Fraunhofer rediscovered them. He noticed that all stars produced different spectral line patterns, but was unable to explain why this occurred.
The solar layer known as the photosphere is responsible for producing the background continuous spectrum, but what causes the dark lines? The intermediate low pressure gases could come from two different sources, ...our own atmosphere or from gasses in the sun's upper atmospheric layers. As a courtesy, all absorption lines produced by gasses in the earth's atmosphere are generally airbrushed out of most books, so you can concentrate on the gases surrounding the sun (or other star).
These spectral absorption lines give astronomers important clues about a star's chemical makeup. For example, the element helium was discovered by noticing that a set of spectral lines of an unknown element appeared in the solar spectrum. It wasn't until later that the same element was found present on the earth. The name helium is derived from the Greek word - Helios (sun).
One technique of discovering planets around other stars relies on a bit of luck and good instruments. If the planet orbits in a plane that causes it to cross our line of sight, there is a small drop in the overall brightness as it passes by (called a transit). If those planets have an atmosphere, we would be able to detect it because new absorption lines would appear in the spectra during the crossing. That is, some of the starlight would have to pass through the planet's atmosphere ...creating dark lines during the transit. The type of absorption lines would identify which elements make up the atmosphere.
Imagine one day astronomers detect a planet with strong lines of oxygen (O2) and ozone (O3). You will conclude that the planet has some form of life which uses photosynthesis as an energy source. Ask a biologist for the details. The discovery of life beyond earth is a very real possibility in your lifetime because astronomers now have the technology. All we need is patience, a little luck, and a life sustaining planet out there.
A transit of Venus across our sun
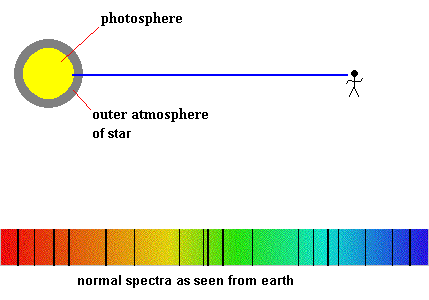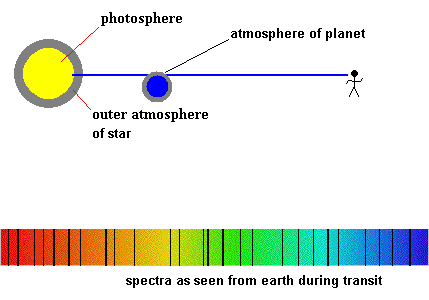
Detecting planetary atmospheres during a transit (animation)
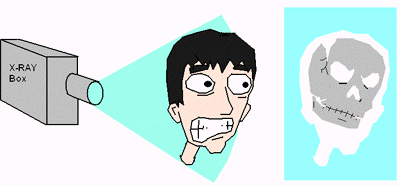
Image by Adam Mihal
Medical X-rays do not make use of reflected waves. Rather, they rely on the difference between transmission and absorption to create images. A normal X-ray is used to see if you have a broken bone because the rays mostly pass through the soft tissue of the body, but are absorbed by bone. A CAT scan is just a 3 dimensional X-ray. Click link 6.4.d if you care to learn more.
Another way to probe with x-rays is by turning down the intensity just enough (about .1% of a medical x-ray) so they penetrate clothing but not skin. The waves reflect (scatter) off the body to produce an image which can be used to detect guns, knives, bombs, etc. This technique called "backscattering" (link 6.4.e) can be used as security in airports, customs, prisons, diamond mines, etc.

Yes, I actually bought these as a kid (they didn't work).
A normal X-ray is where radiation simply passes through the body or is absorbed by denser tissues (bone) ... and recorded (like a photograph) on a plate. An MRI relies on the ability of certain tissues to give off radio signals. This gives doctors clues about tissue composition.
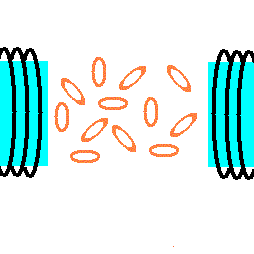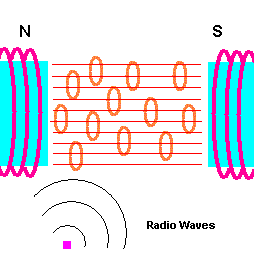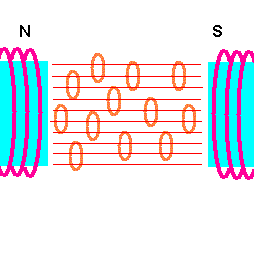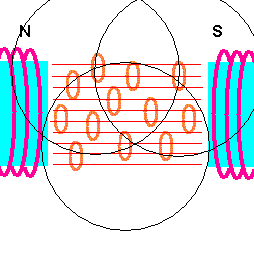
How an MRI works (animation)
The human body is mostly water - H2O! And it is the hydrogen in the water that responds to magnetic fields and radio wave pulses. The first task is to align the hydrogen atoms in your body in an ordered way, ...and this is done by placing your body in an extremely strong magnetic field. Each hydrogen atom acts like a tiny magnet and, therefore, lines up with this external field. The next step is to send pulses of electromagnetic radiation (radio waves) from a different direction. A small percent of hydrogen atoms will turn away from the original magnetic field and align themselves with these incoming radio pulses. The trick is to use a radio frequency which matches the natural oscillation frequency of the atom (this is where the resonance part comes in). The atom then easily absorbs this radio energy. When the pulse is removed, these rebel hydrogen atoms will then re-align with the original magnetic field ... and emit the radio energy they absorbed. Since different tissues have different amounts of water (hydrogen), the amount of radio energy re-emitted varies throughout the body. Surrounding sensors pick up this re-emitted radio energy and produce an image from it. For example, cancer tissue has a lower percent of water compared with healthy tissue so tumors show up as white spots on an MRI.
|
MRI scans Courtesy Wikimedia Commons |
Courtesy Phillips Wikimedia Commons |
X-Rays are also used to study the atomic structure of crystals and metals.
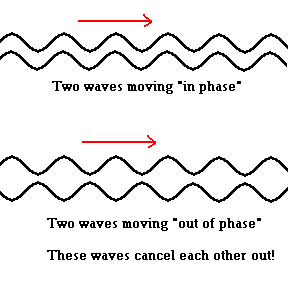
As X-rays strike a crystal surface, they interact with the atoms and are "diffracted" (similar to reflection) off the surface where an interesting thing happens. The waves initially enter the crystal surface "in phase", however, after they interact with the crystal surface they may exit the crystal "out of phase". This leads to a phenomenon known as interference. Two waves that are "in phase" reinforce each other and produce a bright spot (you already know this from the section on lasers). Waves that are "out of phase" undergo destructive interference and cancel each other out! The patterns of wave diffraction give scientists clues about the nature of the crystal structure. That is, by examining the interference patterns diffracted off a crystal, scientists can make educated guesses about the way atoms are arranged within the crystal.
Many scientists believe Rosalind Franklin should have received more credit in the discovery of the double helix structure of DNA. It was her photo 51 (link 6.4.f) that broke the secret.
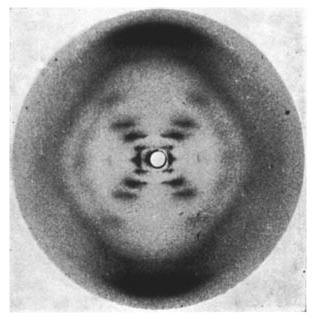
Photo 51 showing the double helix structure of DNA
Source:
Wikimedia Commons
Why use X-rays? All microscopes must deal with some limiting conditions set by the laws of physics. No microscope can resolve objects less than half the wavelength of the radiation used (as the probe). For example, blue light has a wavelength of about .0004 mm. Half of this is .0002 mm. Theoretically, any microscope using blue light cannot resolve two objects closer than .0002 mm. How do you improve the resolution limit? Simple! Use a shorter wavelength. X-rays are relatively easy to make and have an extremely short wavelength.
In 1924, Louis Victor de Broglie suggested that electrons have wave-like properties. The wavelength (λ) is a function of its mass (m) and velocity (v) according to the formula λ = h/mv. This implies that fast moving electrons have a very short corresponding wavelength. As a result, other scientists (including Ernst Ruska) offered the suggestion that high speed electrons could be used as probes in place of X-rays. The electron microscope became a reality in 1931.
Today, the use of electron beams to study the nano-world is routine. Probing beams of electrons are now used in the Scanning Tunneling Microscope (STM), and the Scanning Electron Microscope (SEM). Other techniques to probe atomic surfaces are employed in the Atomic Force Microscope (AFM). Using these revolutionary ideas, individual atoms are revealed and even manipulated. This opens the door for a new field called nanotechnology.
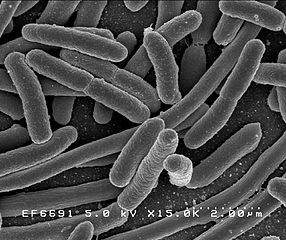
Escherichia coli taken with an Scanning Electron Microscope Courtesy Wikimedia Commons
©2001, 2004, 2007, 2009, 2016,
2018 by Jim Mihal - All rights reserved
No portion may be distributed without the expressed written permission of the author